
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Xanthen-9-one | |
| Other names
9-Oxoxanthene
Diphenyline ketone oxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.816 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H8O2 | |
| Molar mass | 196.205 g·mol−1 |
| Appearance | white solid |
| Melting point | 174 °C (345 °F; 447 K) |
| Sl. sol. in hot water | |
| -108.1·10−6 cm3/mol | |
| Related compounds | |
Related compounds
|
xanthene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Xanthone is an organic compound with the molecular formula O[C6H4]2CO. It is a white solid.
In 1939, xanthone was introduced as an insecticide and it currently finds uses as ovicide for codling moth eggs and as a larvicide.[1] Xanthone is also used in the preparation of xanthydrol, which is used in the determination of urea levels in the blood.[2] It can also be used as a photocatalyst.[3]
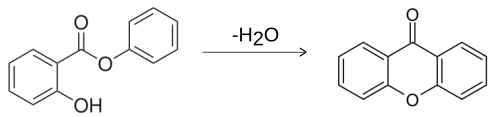
Synthesis
Xanthone can be prepared by the heating of phenyl salicylate:[4]
Xanthone derivatives
The chemical structure of xanthone forms the central core of a variety of naturally occurring organic compounds, such as mangostin, which are sometimes collectively referred to as xanthones or xanthonoids. Over 200 xanthones have been identified. Many xanthones are phytochemicals found in plants in the families Bonnetiaceae, Clusiaceae, Podostemaceae, and others.[5] They are also found in some species of the genus Iris.[6] Some xanthones are found in the pericarp of the mangosteen fruit (Garcinia mangostana) as well as in the bark and timber of Mesua thwaitesii.[7]
Tetrahydroxanthones[edit]
Tetrahydroxanthones are synthesized by various fungi, bacteria, and plants, and are precursors to larger xanthone natural products, such as neosartorin, which is comprised of 5-acetylblennolide A and blennolide C, and exhibits antibacterial activity against Gram-positive bacteria, notably including Staphylococcus aureus.[8][9] It is accepted that chrysophanol is the common intermediate to most if not all tetrahydroxanthones.[10]

In 2018, Yudai Matsuda at City University of Hong Kong, China, discovered the nsr cluster, a polyketide synthase gene responsible for the biosynthesis of neosartorin in A. novofumigatus.[10] However, the enzyme sequence was incomplete until Matsuda and Xingxing Wei established the missing link between anthraquinone chrysophanol and the tetrahydroxanthone scaffolds in 2020.[11] The previously unidentified enzyme, NsrQ, features a catalytic glutamic acid residue in the active site which functions to protonate the enol species produced by NsrK and dearomatize the system.

The biosynthesis of tetrahydroxanthones begins with the synthesis of the anthraquinone chrysophanol (see figure). Once the final reduction and dehydration events take place (NsrJ and NsrI), chrysophanol is accepted into monooxygenase NsrF. A ring opening occurs upon the addition of water to the NsrF product 8 to give 9. NsrG, a methyltransferase, then converts the carboxylic acid into an ester, giving 10 as the product. NsrK, a flavin-dependent monooxygenase, the installs an alcohol ortho- to the ester and methyl groups, breaking the aromaticity of the compound. NsrQ then isomerizes the methyl group to form 12 and 14. The two isomers are then acted upon by either NsrO and CPUR_05418 or just NsrO to give (-)-blennolide B and blennolide A. Further transformation of blennolide A by NsrL yields the precursor natural product 5-actelyblennolide A.

See also
References
- ^ Steiner, L. F. and S. A. Summerland. 1943. Xanthone as an ovicide and larvicide for the codling moth. Journal of Economic Entomology 36, 435-439.
- ^ Bowden, R. S. T. (1962). "The Estimation of Blood Urea by the Xanthydrol Reaction". Journal of Small Animal Practice. 3 (4): 217–218. doi:10.1111/j.1748-5827.1962.tb04191.x.
- ^ Romero, Nathan A.; Nicewicz, David A. (10 June 2016). "Organic Photoredox Catalysis". Chemical Reviews. 116 (17): 10075–10166. doi:10.1021/acs.chemrev.6b00057. PMID 27285582.
- ^ A. F. Holleman (1927). "Xanthone". Org. Synth. 7: 84. doi:10.15227/orgsyn.007.0084.
- ^ "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II". Botanical Journal of the Linnean Society. 141 (4): 399–436. 2003. doi:10.1046/j.1095-8339.2003.t01-1-00158.x.
- ^ Williams, C.A; Harborne, J.B.; Colasante, M. (2000). "The pathway of chemical evolution in bearded iris species based on flavonoid and xanthone patterns" (PDF). Annali di Botanica. 58: 51–54. Retrieved 28 October 2015.
- ^ Bandaranayake, Wickramasinghe M.; Selliah, Sathiaderan S.; Sultanbawa, M.Uvais S.; Games, D.E. (1975). "Xanthones and 4-phenylcoumarins of Mesua thwaitesii". Phytochemistry. 14: 265–269. doi:10.1016/0031-9422(75)85052-7.
- ^ Matsuda, Yudai; Gotfredsen, Charlotte H.; Larsen, Thomas O. (2018-11-16). "Genetic Characterization of Neosartorin Biosynthesis Provides Insight into Heterodimeric Natural Product Generation". Organic Letters. 20 (22): 7197–7200. doi:10.1021/acs.orglett.8b03123. ISSN 1523-7060.
- ^ Ola, Antonius R.B.; Debbab, Abdessamad; Aly, Amal H.; Mandi, Attila; Zerfass, Ilka; Hamacher, Alexandra; Kassack, Matthias U.; Brötz-Oesterhelt, Heike; Kurtan, Tibor; Proksch, Peter (2014-01). "Absolute configuration and antibiotic activity of neosartorin from the endophytic fungus Aspergillus fumigatiaffinis". Tetrahedron Letters. 55 (5): 1020–1023. doi:10.1016/j.tetlet.2013.12.070.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Greco, Claudio; de Mattos-Shipley, Kate; Bailey, Andrew M.; Mulholland, Nicholas P.; Vincent, Jason L.; Willis, Christine L.; Cox, Russell J.; Simpson, Thomas J. (2019). "Structure revision of cryptosporioptides and determination of the genetic basis for dimeric xanthone biosynthesis in fungi". Chemical Science. 10 (10): 2930–2939. doi:10.1039/C8SC05126G. ISSN 2041-6520. PMC 6428139. PMID 30996871.
{{cite journal}}: no-break space character in|first3=at position 7 (help)CS1 maint: PMC format (link) - ^ Wei, Xingxing; Matsuda, Yudai (2020-03-06). "Unraveling the Fungal Strategy for Tetrahydroxanthone Biosynthesis and Diversification". Organic Letters. 22 (5): 1919–1923. doi:10.1021/acs.orglett.0c00285. ISSN 1523-7060.