Academic Challenger (talk | contribs) m Reverted edits by 117.47.212.225 (talk) to last version by SteveBaker |
|||
| Line 99: | Line 99: | ||
The claimed applications of HHO and Aquygen are practically indistinguishable from the original claims of Yull Brown. The HHO trademark is associated with an unproven state of matter called ''magnegases'', and a discredited theory about [[magnecule]]s,<ref>{{cite journal |
The claimed applications of HHO and Aquygen are practically indistinguishable from the original claims of Yull Brown. The HHO trademark is associated with an unproven state of matter called ''magnegases'', and a discredited theory about [[magnecule]]s,<ref>{{cite journal |
||
| title = Comments on “A new gaseous and combustible form of water,” by R.M. Santilli (Int. J. Hydrogen Energy 2006: 31(9), 1113–1128) | journal = International Journal of Hydrogen Energy | issue = 32 | pages = p. 1309–1312 | author = J. M. Calo |date=November 3, 2006 }}{{doi|10.1016/j.ijhydene.2006.11.004}}</ref> which is the basis for a number of fraudulent claims, and third party [[water-fuelled car]] scam attempts. |
| title = Comments on “A new gaseous and combustible form of water,” by R.M. Santilli (Int. J. Hydrogen Energy 2006: 31(9), 1113–1128) | journal = International Journal of Hydrogen Energy | issue = 32 | pages = p. 1309–1312 | author = J. M. Calo |date=November 3, 2006 }}{{doi|10.1016/j.ijhydene.2006.11.004}}</ref> which is the basis for a number of fraudulent claims, and third party [[water-fuelled car]] scam attempts. |
||
=====Gasoline Additive==== |
|||
However, that does not diminish the very real value of HHO gas a gasoline additive which commonly can save 25% to 50% of fuel consumption over gasoline used alone in most vehicles. That discovery has ignited thousands of new HHO designers whose videos of their projects are multiplying by the minute on YouTube. While some of the devices produce savings and some may not, many drivers are learning that adding HHO gas via an electrolyzer, bubbler and hose to the intake manifold will sharply increase gasoline mileage, clean engine "gunk" and reduce pollutants, as the gas burns far cleaner and hotter than gasoline. |
|||
However, unless the gas is used judiciously, such as on longer trips rather than in-city, stop-and-go traffice, the draw on the battery to power the electrolyzer can discharge the battery and eventually degrade the alternator. Most users of HHO gas therefore employ a dashboard switch to turn the mixture on and off at the manifold, allowing the battery to quickly recharge while using only gasoline. |
|||
====HHO Games & Exposition==== |
|||
The <A HREF="http://www.hhoexpo.com">First Annual HHO Games & Exposition</A HREF>, known as HHO Expo, is scheduled to take place in Bradenton, Fla., on Nov. 11-14, 2008, to bring together designers, inventors and fabricators and provide public demonstrations on a wide variety of veicles of the gasoline savings achieved. The HHO Expo plans to present a $100,000 Grand Prize for the Best of Show. The organizers also hope to begin the process of setting standards fore simple, inexpensive HHO Gas kits in conventional automobiles, trucks and other conveyances. White House officials, academic experts and representatives of indutry leaders are expected to participate in seminars, workshops, speeches and vehicle demonstrations. An industry trade group is being formed to improve the industry's visibility. Industry leaders will meet at a pre-Expo invitation-only event in Bradenton in August, 2008. The organizer is Joe Shea, a well-known online newspaper editor. |
|||
== References == |
== References == |
||
Revision as of 03:21, 9 June 2008
Oxyhydrogen is a mixture of hydrogen and oxygen gases, typically in a 2:1 atomic ratio, the same proportion as water.[1] This gaseous mixture is widely used for torches for the processing of refractory materials.[citation needed]
Properties
Oxyhydrogen will combust when brought to its autoignition temperature. For a stoichiometric mixture at normal atmospheric pressure, autoignition occurs at about 570 °C (1065 °F).[2] The minimum energy required to ignite such a mixture with a spark is about 20 microjoules.[2] At normal temperature and pressure, oxyhydrogen can burn when it is between about 4% and 94% hydrogen by volume.[2]
When ignited, the gas mixture converts to water vapor and releases energy, which sustains the reaction: 241.8 kJ of energy (LHV) for every mole of Template:Hydrogen burned. The amount of heat energy released is independent of the mode of combustion, but the temperature of the flame varies.[1] The maximum temperature of about 2800 °C is achieved with a pure stoichiometric mixture, about 700 degrees hotter than a hydrogen flame in air.[3][4][5] When either of the gases is mixed in excess of this ratio, or when mixed with an inert gas like nitrogen, the heat must spread throughout a greater quantity of matter and the temperature will be lower.[1]
Applications

Lighting
Many forms of oxyhydrogen lamps have been described, such as the limelight, which used an oxyhydrogen flame to heat a piece of lime to white hot incandescence.[6] Because of the explosiveness of the oxyhydrogen, limelights have been replaced by electric lighting.
It was much used in platinum works,[citation needed] as platinum could be melted (at a temperature of 1768.3 °C) only in an oxyhydrogen flame, or an electric furnace (which is now used instead).
Oxyhydrogen torch
An oxyhydrogen torch is an oxy-gas torch, which burns hydrogen (the fuel) with oxygen (the oxidizer). It is used for cutting and welding metals, glass, and thermoplastics.[6] An oxyhydrogen torch is used in the glass industry for "fire polishing"; slightly melting the surface of glass to remove scratches and dullness.[citation needed]
The oxyhydrogen flame begins a short distance from the torch tip; if the distance is great enough the torch tip can remain relatively cool.[7]
Production
A pure stoichiometric mixture is most easily obtained by water electrolysis, which uses an electric current to dissociate the water molecules:
- electrolysis: 2 H2O → 2 H2 + O2
- combustion: 2 H2 + O2 → 2 H2O
The energy required to generate the oxyhydrogen always exceeds the energy released by combusting it. (See Electrolysis of water:Efficiency).
Water torch
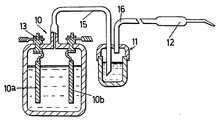
A water torch is a kind of oxyhydrogen torch, that is fed by oxygen and hydrogen generated on demand by water electrolysis. The device avoids the need for bottled oxygen and hydrogen, and requires electricity. Some models of water torches mix the two gases immediately after production (vs. the torch tip) making the gas mixture more accurate.[8] This electrolyzer design is referred to as "common-ducted",[7] and the first was invented by William A. Rhodes in 1966.[9] Water torches must be designed to mitigate flashback by strengthening the electrolytic chamber. Use of an intermediary water bubbler eliminates potential electrolyzer damage from flashback, with a dry flashback arrestor being ineffective due to flame velocity. The bubbler is connected directly in series with the output gas. A water bubbler is sometimes referred to as a wet flashback arrestor, and effectively captures any remaining electrolyte in the output gas. Suitable electrolytes include sodium or potassium hydroxide, and other salts that ionize well.[7] Also "the electrolyzer system must be of high enough pressure to keep the gas velocity at the nozzle above the combustion velocity of the flame, or the system will backfire".[7] For images of water torch equipment see these links: [1] [2] [3][4][5][6][7][8].

Brown's design
Oxyhydrogen gas produced in a common-ducted electrolyzer has been referred to as "Brown's gas",[citation needed] after Yull Brown who received a utility patent for a series cell common-ducted electrolyzer in 1977 and 1978 (the term "Brown's gas" is not used in his patents, but "a mixture of oxygen and hydrogen" is referenced).[8][10] Brown's torches also used an electric arc to increase the temperature of the flame (called atomic welding):[8]
Aquygen
The firm Hydrogen Technology Applications (HTA) has trademarked oxyhydrogen as Aquygen. HTA's founder Dennis Klein holds a patent for an electrolyzer design which states that it differs from Yull Brown's torch patents in that it lacks an electric arc feature.[11]
The claimed applications of HHO and Aquygen are practically indistinguishable from the original claims of Yull Brown. The HHO trademark is associated with an unproven state of matter called magnegases, and a discredited theory about magnecules,[12] which is the basis for a number of fraudulent claims, and third party water-fuelled car scam attempts.
=Gasoline Additive
However, that does not diminish the very real value of HHO gas a gasoline additive which commonly can save 25% to 50% of fuel consumption over gasoline used alone in most vehicles. That discovery has ignited thousands of new HHO designers whose videos of their projects are multiplying by the minute on YouTube. While some of the devices produce savings and some may not, many drivers are learning that adding HHO gas via an electrolyzer, bubbler and hose to the intake manifold will sharply increase gasoline mileage, clean engine "gunk" and reduce pollutants, as the gas burns far cleaner and hotter than gasoline.
However, unless the gas is used judiciously, such as on longer trips rather than in-city, stop-and-go traffice, the draw on the battery to power the electrolyzer can discharge the battery and eventually degrade the alternator. Most users of HHO gas therefore employ a dashboard switch to turn the mixture on and off at the manifold, allowing the battery to quickly recharge while using only gasoline.
HHO Games & Exposition
The <A HREF="http://www.hhoexpo.com">First Annual HHO Games & Exposition</A HREF>, known as HHO Expo, is scheduled to take place in Bradenton, Fla., on Nov. 11-14, 2008, to bring together designers, inventors and fabricators and provide public demonstrations on a wide variety of veicles of the gasoline savings achieved. The HHO Expo plans to present a $100,000 Grand Prize for the Best of Show. The organizers also hope to begin the process of setting standards fore simple, inexpensive HHO Gas kits in conventional automobiles, trucks and other conveyances. White House officials, academic experts and representatives of indutry leaders are expected to participate in seminars, workshops, speeches and vehicle demonstrations. An industry trade group is being formed to improve the industry's visibility. Industry leaders will meet at a pre-Expo invitation-only event in Bradenton in August, 2008. The organizer is Joe Shea, a well-known online newspaper editor.
References
- ^ a b c 1911 Encyclopedia. "Oxyhydrogen Flame". (Available here Accessed 2008-01-19.)
- ^ a b c O'Connor, Ken. "Hydrogen". NASA Glenn Research Center Glenn Safety Manual.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Calvert, Dr. James B. (2006-09-09). "Hydrogen". University of Denver faculty page. Retrieved 2008-04-05. "An air-hydrogen torch flame reaches 2045 °C, while an oxyhydrogen flame reaches 2660 °C."
- ^ "Adiabatic Flame Temperature". The Engineering Toolbox. Retrieved 2008-04-05. "Oxygen as Oxidizer: 3079 K, Air as Oxidizer: 2384 K"
- ^ "Temperature of a Blue Flame". Retrieved 2008-04-05. "Hydrogen in air: 2,400 K, Hydrogen in Oxygen: 3,080 K"
- ^ a b William Augustus Tilden. Chemical Discovery and Invention in the Twentieth Century. Adamant Media Corporation. p. 80. ISBN 0543916464.
- ^ a b c d George Wiseman. Brown's Gas Book 2. Eagle Research. p. 59. ISBN 1895882192.
- ^ a b c d e US patent 4014777, Yull Brown, "Welding", issued 1977-03-29
- ^ US patent 3262872, William Rhodes, "Generator Patent", issued 1966-7-26
- ^ US patent 4081656, Yull Brown, "Arc-assisted oxy/hydrogen welding", issued 1978-3-28
- ^ US patent 6,689,259, Dennis Klein, "Mixed gas generator", issued 2004-2-10
- ^ J. M. Calo (November 3, 2006). "Comments on "A new gaseous and combustible form of water," by R.M. Santilli (Int. J. Hydrogen Energy 2006: 31(9), 1113–1128)". International Journal of Hydrogen Energy (32): p. 1309–1312.
{{cite journal}}:|pages=has extra text (help)doi:10.1016/j.ijhydene.2006.11.004
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press. {{cite encyclopedia}}: Missing or empty |title= (help)