Content deleted Content added
No edit summary |
172.58.203.73 (talk) No edit summary Tag: review edit |
||
| (38 intermediate revisions by 24 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
| Watchedfields = changed |
|||
| ⚫ | |||
| verifiedrevid = 448571173 |
|||
| ImageSize = 150px |
|||
| ⚫ | |||
| IUPACName = 2,6-dioxo-1,3,4,5,7,8-hexanitrodecahydro-1H,5H-diimidazo[4,5-b:4',5'-e]pyrazine |
|||
| |
| ImageSize = 200px |
||
| PIN = 1,3,4,5,7,8-Hexanitrooctahydrodiimidazo[4,5-''b'':4′,5′-''e'']pyrazine-2,6(1''H'',3''H'')-dione |
|||
| ⚫ | |||
| OtherNames = Hexanitrohexaazatricyclododecanedione <br> DTNGU <br> Naza/Namsorguyl/uryl HnHaza/amTcDglcDuryl |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 23078717 |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C6H4N12O14/c19-5-9(15(25)26)1-2(10(5)16(27)28)8(14(23)24)4-3(7(1)13(21)22)11(17(29)30)6(20)12(4)18(31)32/h1-4H |
|||
| InChI=1/C6H4N12O14/c19-5-9(15(25)26)1-2(10(5)16(27)28)8(14(23)24)4-3(7(1)13(21)22)11(17(29)30)6(20)12(4)18(31)32/h1-4H |
|||
| InChIKey = ZFBXJPJQJKATGM-UHFFFAOYAE |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = ZFBXJPJQJKATGM-UHFFFAOYSA-N |
|||
}} |
}} |
||
| |
|Section2={{Chembox Properties |
||
| C=6|H=4|N=12|O=14 |
|||
| Formula = C<sub>6</sub>H<sub>4</sub>N<sub>12</sub>O<sub>14</sub> |
|||
| |
| Appearance = |
||
| |
| Density = |
||
| |
| MeltingPt = |
||
| |
| BoilingPt = |
||
| |
| Solubility = |
||
| Solubility = |
|||
}} |
}} |
||
| |
|Section3={{Chembox Hazards |
||
| |
| MainHazards = |
||
| |
| FlashPt = |
||
| |
| AutoignitionPt = |
||
}} |
}} |
||
| |
|Section6={{Chembox Explosive |
||
| ShockSens = |
| ShockSens = |
||
| FrictionSens = |
| FrictionSens = |
||
| |
| DetonationV = 9700 m/s |
||
}} |
}} |
||
}} |
}} |
||
'''HHTDD''' (''' |
'''HHTDD''' ('''hexanitrohexaazatricyclododecanedione''') is a powerful but moisture sensitive [[explosive]] compound. It is essentially an open analogue of the cyclic nitroamine cage compounds such as [[CL-20]]. While it is highly explosive, with a [[velocity of detonation]] even higher than that of CL-20, HHTDD readily decomposes in the presence of even trace amounts of water, making it unsuitable for any practical applications.<ref>{{cite journal|doi =10.1021/jo00010a043|title =Facile synthesis and nitration of cis-syn-cis-2,6-dioxodecahydro-1H,5H-diimidazo[4,5-b:4',5'-e]pyrazine|year =1991|last1 =Vedachalam|first1 =Murugappa|last2 =Ramakrishnan|first2 =Vayalakkavoor T.|last3 =Boyer|first3 =Joseph H.|last4 =Dagley|first4 =Ian J.|last5 =Nelson|first5 =Keith A.|last6 =Adolph|first6 =Horst G.|last7 =Gilardi|first7 =Richard|last8 =George|first8 =Clifford|last9 =Flippen-Anderson|first9 =Judith L.|journal =The Journal of Organic Chemistry|volume =56|issue =10|pages =3413–3419}}</ref> |
||
==See also== |
|||
*[[TNGU]] |
|||
*[[2,4,6-Tris(trinitromethyl)-1,3,5-triazine]] |
|||
*[[4,4’-Dinitro-3,3’-diazenofuroxan]] (DDF) |
|||
*[[Heptanitrocubane]] |
|||
*[[Octanitrocubane]] |
|||
*[[RE factor]] |
|||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
{{DEFAULTSORT:Hhtdd}} |
|||
[[Category:Explosive chemicals]] |
[[Category:Explosive chemicals]] |
||
[[Category:Nitroamines]] |
|||
Latest revision as of 01:44, 25 June 2022
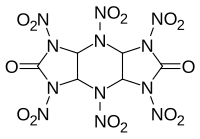
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,4,5,7,8-Hexanitrooctahydrodiimidazo[4,5-b:4′,5′-e]pyrazine-2,6(1H,3H)-dione | |
| Other names
Hexanitrohexaazatricyclododecanedione
DTNGU Naza/Namsorguyl/uryl HnHaza/amTcDglcDuryl | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H4N12O14 | |
| Molar mass | 468.168 g·mol−1 |
| Explosive data | |
| Detonation velocity | 9700 m/s |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
HHTDD (hexanitrohexaazatricyclododecanedione) is a powerful but moisture sensitive explosive compound. It is essentially an open analogue of the cyclic nitroamine cage compounds such as CL-20. While it is highly explosive, with a velocity of detonation even higher than that of CL-20, HHTDD readily decomposes in the presence of even trace amounts of water, making it unsuitable for any practical applications.[1]
See also[edit]
- TNGU
- 2,4,6-Tris(trinitromethyl)-1,3,5-triazine
- 4,4’-Dinitro-3,3’-diazenofuroxan (DDF)
- Heptanitrocubane
- Octanitrocubane
- RE factor
References[edit]
- ^ Vedachalam, Murugappa; Ramakrishnan, Vayalakkavoor T.; Boyer, Joseph H.; Dagley, Ian J.; Nelson, Keith A.; Adolph, Horst G.; Gilardi, Richard; George, Clifford; Flippen-Anderson, Judith L. (1991). "Facile synthesis and nitration of cis-syn-cis-2,6-dioxodecahydro-1H,5H-diimidazo[4,5-b:4',5'-e]pyrazine". The Journal of Organic Chemistry. 56 (10): 3413–3419. doi:10.1021/jo00010a043.