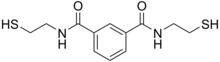
| |
| Names | |
|---|---|
| IUPAC name
N,N′-bis(2-mercaptoethyl)isophthalamide
| |
| Other names
BDET; BDTH2; BDETH2; N,N′-Bis(2-mercaptoethyl)-1,3-benzenedicarboxamide; N1,N3-bis(2-mercaptoethyl)isophthalamide; NBMI;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | 1,3-benzenediamidoethanethiol |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H16N2O2S2 | |
| Molar mass | 284.39 g·mol−1 |
| Density | 1.23 g/mL |
| Melting point | 132 to 135 °C (270 to 275 °F; 405 to 408 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
BDTH2 (also called BDET and BDETH2; trade names B9, MetX and OSR#1) is an organosulfur compound that is used as a chelation agent.[2] It is a colourless solid. The molecule consists of two thiol groups and linked via a pair of amide groups. Due to its lipid solubility it appears to be of limit use.[3] Sold as an nutritional supplement and antioxidant from 2006-2010 in the US, the Food and Drug Administration (FDA) stopped its marketing until it has undergone full drug approval process. The manufacturer filed for its use as a chelator in clinical trials of mercury poisoning. The compound is banned in the US as a treatment of autism,[4] despite a marketing campaign aimed at parents of children with this disorder.
Preparation
This compound is prepared by treating cysteamine with isophthaloyl dichloride to give the desired amide:[1][2]
Use
Environmental remediation
BDTH2 can be used to chelate heavy metals like lead, cadmium, copper, manganese, zinc, iron und mercury from ground water, coal tailings, gold ore, waste water of battery-recycling plants or contaminated soil.[2]
BDTH2 appears to bind mercury more strongly than other chelators. Even at high pH and the presence of cyanides, as in waste water of gold mines, mercury does not dissociate. The particular stability of the mercury bond can be attributed to the linear position of the two thiols.[5] A company named Covalent Research Technologies tried removal of mercury from fluegas using BDTH2, which was not cost effective, and is working on water filtration to remove arsenic and mercury.[6]
Clinical use
Animal experiments with inorganic mercury showed, that BDTH2 effectively binds mercury in the body, but it is not excreted. Experimental animals showed no signs of poisoning. It is unclear, how the BDTH2-mercury-chelate behaves in the long term. BDTH2 is lipophilic, as opposed to DMPS and DMSA and thus cannot enter tissues. It can cross the blood-brain-barrier and enters the bone marrow.[6] In animal experiments, the amount of mercury in brain tissue was not increased, but also not decreased. However there are indications that the BDTH2-mercury-compound moves into adipose tissue.[3] It is unknown, how BDTH2 works with methyl-mercury. The ability to bind copper and zinc in vivo appears too weak, as opposed to DMPS und DMSA, while iron is probably bound. It has no affinity to Ca2+, Mg2+, Na+ und K+.[3]
Until July 2010 CTI Science sold BDTH2 under the name OSR#1 as a nutritional supplement.[7] Since OSR#1 didn't fulfill criteria of a nutritional supplement, its sale was stopped under pressure of the U.S. Food and Drug Administration.[8] In January 2012 BDTH2 was approved by the European Commission as an orphan drug, which guarantees CTI Science 10 years of exclusive marketing rights.[9]
Potential applications
Like most thiols, BDTH2 binds to mercury salts to form thiolate complexes. In principle, it could be used to remove mercury from water for industrial applications under a wide range of conditions, including the high pH and cyanide of the effluent from gold mining. In industrial use, BDTH2 is easy to make, does not form disulfides, and can be used either as-is or in the form of sodium or potassium salts that are more soluble in water.[1]
BDTH2 binds to mercury with a strong, nonpolar covalent bond within a water-insoluble organic framework. The resulting BDT–Hg precipitate is stable, and leaches mercury only under highly acidic or basic conditions. BDTH2 also binds to other elements, including arsenic, cadmium, copper, lead, and selenium.[1] It is effective and economical for removing small traces of mercury from polluted soil, as the precipitate is inert and can be left in the soil after treatment.[10]
Dietary supplement and controversy
Despite the fact that chelation therapy has not been shown to have a beneficial effect,[11] BDTH2 had been marketed under the name OSR#1 as a dietary supplement for treatment of autism.[4] The U.S. Food and Drug Administration determined that BDTH2 is a drug rather than a supplement and issued a warning,[12][13] resulting in its removal from the market.[14] The main proponent of the compound, Dr. Boyd Haley, was chairman of the department of chemistry where research is also conducted on the utility of this compound for remediation of heavy metal pollution.[4][1]
History
When David Atwood, chemistry professor at University of Kentucky, learnt that there was no industrial chelator for mercury in about 1994, he screened more than 50 compounds until finding BDTH2. He licensed it in 2006 and started the company CTI Science with the long term goal of using BDTH2 in mercury poisoning. Atwood conducted rat experiments demonstrating safety and efficacy in induced mercury poisoning and filed a premarket authorization with the FDA in 2008. A contract manufacturer increased the yield of synthesis, eliminated chloroform as a solvent and isolated BDTH2 in powder form for capsules. After FDA stopped sales as a dietary supplement in 2010, Atwood filed for Investigational Medicinal Product Dossier (equivalent to Investigational New Drug) with the European Medicines Agency. Complying with Good Manufacturing Practices meant removing impurities closely resembling NMBI, as well as its dimers and trimers, which was helped by using HPLC for analysis. Atwood expects to begin clinical trials at the end of 2014 in Sweden.[6]
See also
References
- ^ a b c d e Blue LY, Jana P, Atwood DA. Aqueous mercury precipitation with the synthetic dithiolate, BDTH2. Fuel. June 2010;89(6):1326-1330. doi:10.1016/j.fuel.2009.10.031.
- ^ a b c Blue LY, Immobilizatin of mercury and arsenic through covalent thiolate bonding for the purpose of environmental remediation, Dissertation, University of Kentucky, 2010
- ^ a b c Clarke D, Efficacy of a Novel Chelator for Mercury Chelation and Distribution, Dissertation, Arkansas State University, December 2012
- ^ a b c Tsouderos T. OSR#1: industrial chemical or autism treatment? Chicago Tribune. 2010-01-17.
- ^ Lisa Y. Blue, Mike A. Van Aelstyn, Matthew Matlock, David A. Atwood, Low-level mercury removal from groundwater using a synthetic chelating ligand, Water Research, Vol. 42, Issues 8-9, April 2008, pp2025-2028
- ^ a b c Mullin, Rick (3 March 2014). "A mercury chelator". C&EN Online. 92 (9): 18–19.
{{cite journal}}:|access-date=requires|url=(help) - ^ Forrest Health Online: Archived (Date missing) at forresthealth.com (Error: unknown archive URL)
- ^ Warning letter CIN-10-107927-14 17 June 2010 FDA to CTI Science Inc.
- ^ EU/3/11/944 (PDF; 112 kB) NBMI as orphan drug by the EC
- ^ Blue LY, Van Aelstyn MA, Matlock M, Atwood DA. Low-level mercury removal from groundwater using a synthetic chelating ligand. Water Res. 2008;42(8–9):2025–8. doi:10.1016/j.watres.2007.12.010.
- ^ Weber, W; Newmark, S (2007). "Complementary and alternative medical therapies for attention-deficit/hyperactivity disorder and autism". Pediatric clinics of North America. 54 (6): 983–1006, xii. doi:10.1016/j.pcl.2007.09.006. PMID 18061787.
- ^ Tsouderos, Trine (June 23, 2010). "FDA warns maker of product used as alternative autism treatment". Chicago Tribune. Archived from the original on 30 July 2010. Retrieved July 30, 2010.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Warning Letter CIN-10-107927-14". Inspections, Compliance, Enforcement, and Criminal Investigations. U.S Department of Health and Human Services / Food and Drug Administration. June 17, 2010. Archived from the original on 27 June 2010. Retrieved June 28, 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Tsouderos, Trine (July 26, 2010). "Controversial supplement to come off shelves". Chicago Tribune. Archived from the original on 30 July 2010. Retrieved July 30, 2010.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
