m Scanned 3 urls; found 3 archives (3 in CiteWeb Templates; 1 in References). See User:DASHBot/Dead Links for settings, shutoff, info, questions. |
180.252.25.137 (talk) No edit summary |
||
| Line 27: | Line 27: | ||
| C=12 | H=16 | N=2 | O=2 | S=2 |
| C=12 | H=16 | N=2 | O=2 | S=2 |
||
| Appearance = |
| Appearance = |
||
| Density = |
| Density = 1.23 g/mL |
||
| MeltingPt = 132–135 °C<ref name=Blue/> |
| MeltingPt = 132–135 °C<ref name=Blue/> |
||
| BoilingPt = |
| BoilingPt = |
||
Revision as of 02:53, 2 January 2013
| |
| Names | |
|---|---|
| IUPAC name
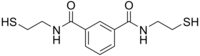
N,N′-bis(2-mercaptoethyl)isophthalamide
| |
| Other names
BDET; BDETH2; N,N′-Bis(2-mercaptoethyl)-1,3-benzenedicarboxamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | 1,3-benzenediamidoethanethiol |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H16N2O2S2 | |
| Molar mass | 284.39 g·mol−1 |
| Density | 1.23 g/mL |
| Melting point | 132–135 °C[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
BDTH2 (also called BDET and BDETH2; trade names B9, MetX and OSR#1) is an organosulfur compound that is used as a chelation agent. It is a colourless solid. The molecule consists of two thiol groups and linked via a pair of amide groups. The compound is banned in the US as a treatment of autism,[2] despite a marketing campaign aimed at parents of children with this disorder.
Preparation
This compound is prepared by treating cysteamine with isophthaloyl dichloride to give the desired amide:[1]
Potential applications
Like most thiols, BDTH2 binds to mercury salts to form thiolate complexes. In principle, it could be used to remove mercury from water for industrial applications under a wide range of conditions, including the high pH and cyanide of the effluent from gold mining. In industrial use, BDTH2 is easy to make, does not form disulfides, and can be used either as-is or in the form of sodium or potassium salts that are more soluble in water.[1]
BDTH2 binds to mercury with a strong, nonpolar covalent bond within a water-insoluble organic framework. The resulting BDT–Hg precipitate is stable, and leaches mercury only under highly acidic or basic conditions. BDTH2 also binds to other elements, including arsenic, cadmium, copper, lead, and selenium.[1] It is effective and economical for removing small traces of mercury from polluted soil, as the precipitate is inert and can be left in the soil after treatment.[3]
Dietary supplement and controversy
Despite the fact that chelation therapy has not been shown to have a beneficial effect,[4] BDTH2 had been marketed under the name OSR#1 as a dietary supplement for treatment of autism.[2] The U.S. Food and Drug Administration determined that BDTH2 is a drug rather than a supplement and issued a warning,[5][6] resulting in its removal from the market.[7] The main proponent of the compound, Dr. Boyd Haley, was chairman of the department of chemistry where research is also conducted on the utility of this compound for remediation of heavy metal pollution.[2][1]
References
- ^ a b c d e Blue LY, Jana P, Atwood DA. Aqueous mercury precipitation with the synthetic dithiolate, BDTH2. Fuel. 2009. doi:10.1016/j.fuel.2009.10.031.
- ^ a b c Tsouderos T. OSR#1: industrial chemical or autism treatment? Chicago Tribune. 2010-01-17.
- ^ Blue LY, Van Aelstyn MA, Matlock M, Atwood DA. Low-level mercury removal from groundwater using a synthetic chelating ligand. Water Res. 2008;42(8–9):2025–8. doi:10.1016/j.watres.2007.12.010.
- ^ Weber, W; Newmark, S (2007). "Complementary and alternative medical therapies for attention-deficit/hyperactivity disorder and autism". Pediatric clinics of North America. 54 (6): 983–1006, xii. doi:10.1016/j.pcl.2007.09.006. PMID 18061787.
- ^ Tsouderos, Trine (June 23, 2010). "FDA warns maker of product used as alternative autism treatment". Chicago Tribune. Archived from the original on 30 July 2010. Retrieved July 30, 2010.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Warning Letter CIN-10-107927-14". Inspections, Compliance, Enforcement, and Criminal Investigations. U.S Department of Health and Human Services / Food and Drug Administration. June 17, 2010. Archived from the original on 27 June 2010. Retrieved June 28, 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Tsouderos, Trine (July 26, 2010). "Controversial supplement to come off shelves". Chicago Tribune. Archived from the original on 30 July 2010. Retrieved July 30, 2010.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
