No edit summary |
m Journal cites, added 1 PMID using AWB (11793) |
||
| Line 1: | Line 1: | ||
{{ |
{{other uses|Ampelopsin (compounds)}} |
||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Line 40: | Line 40: | ||
'''Ampelopsin''', also known as '''dihydromyricetin''', is a [[flavanonol]], a type of flavonoid. It is found in the ''[[Ampelopsis]]'' species ''japonica'', ''megalophylla'', and ''grossedentata''; ''[[Cercidiphyllum japonicum]]''; ''[[Hovenia dulcis]]''; ''[[Rhododendron cinnabarinum]]''; some ''[[Pinus]]'' species; and some ''[[Cedrus]]'' species,<ref name="EncTCM">{{cite book|title = Encyclopedia of Traditional Chinese Medicines – Molecular Structures, Pharmacological Activities, Natural Sources and Applications: Vol. 1: Isolated Compounds A-C|url = https://books.google.se/books?id=PMsXJnUYTFkC&printsec=frontcover&dq=isbn:9783642167355&hl=en&sa=X&ei=9biiVcOTOOGqygODs42IDQ&redir_esc=y#v=onepage&q&f=false|publisher = Springer Science & Business Media|date = 2011-02-21|isbn = 978-3-642-16735-5|first = Jiaju|last = Zhou|first2 = Guirong|last2 = Xie|first3 = Xinjian|last3 = Yan}}</ref> as well as in ''[[Salix sachalinensis]]''.<ref>{{cite journal|title = A Journey of Twenty-Five Years through the Ecological Biochemistry of Flavonoids|url = http://dx.doi.org/10.1271/bbb.70028|journal = Bioscience, Biotechnology, and Biochemistry|date = June 23, 2007|issn = 0916-8451|pmid = 17587669|pages = 1387–1404|volume = 71|issue = 6|doi = 10.1271/bbb.70028|first = Satoshi|last = Tahara}}</ref> |
'''Ampelopsin''', also known as '''dihydromyricetin''', is a [[flavanonol]], a type of flavonoid. It is found in the ''[[Ampelopsis]]'' species ''japonica'', ''megalophylla'', and ''grossedentata''; ''[[Cercidiphyllum japonicum]]''; ''[[Hovenia dulcis]]''; ''[[Rhododendron cinnabarinum]]''; some ''[[Pinus]]'' species; and some ''[[Cedrus]]'' species,<ref name="EncTCM">{{cite book|title = Encyclopedia of Traditional Chinese Medicines – Molecular Structures, Pharmacological Activities, Natural Sources and Applications: Vol. 1: Isolated Compounds A-C|url = https://books.google.se/books?id=PMsXJnUYTFkC&printsec=frontcover&dq=isbn:9783642167355&hl=en&sa=X&ei=9biiVcOTOOGqygODs42IDQ&redir_esc=y#v=onepage&q&f=false|publisher = Springer Science & Business Media|date = 2011-02-21|isbn = 978-3-642-16735-5|first = Jiaju|last = Zhou|first2 = Guirong|last2 = Xie|first3 = Xinjian|last3 = Yan}}</ref> as well as in ''[[Salix sachalinensis]]''.<ref>{{cite journal|title = A Journey of Twenty-Five Years through the Ecological Biochemistry of Flavonoids|url = http://dx.doi.org/10.1271/bbb.70028|journal = Bioscience, Biotechnology, and Biochemistry|date = June 23, 2007|issn = 0916-8451|pmid = 17587669|pages = 1387–1404|volume = 71|issue = 6|doi = 10.1271/bbb.70028|first = Satoshi|last = Tahara}}</ref> |
||
''[[Hovenia dulcis]]'' has been used in traditional [[Kampo|Japanese]], [[Traditional chinese medicine|Chinese]], and [[Traditional Korean medicine|Korean]] medicines to treat fever, parasitic infection, as a laxative, and a treatment of liver diseases, and as a [[hangover]] treatment.<ref name=Hyun>{{cite journal|last1=Hyun|first1=Tae|last2=Eom|first2=Seung|last3=Yu|first3=Chang|last4=Roitsch|first4=Thomas|title=Hovenia dulcis– An Asian Traditional Herb|journal=Planta Medica|volume=76|issue=10|year=2010|pages=943–949|issn=0032-0943|doi=10.1055/s-0030-1249776}}</ref> Methods have been developed to extract ampelopsin from it at large scales, and laboratory research has been conducted with the compound to see if it might be useful as a drug in any of the conditions for which the parent plant has been traditionally used.<ref name=Hyun /> |
''[[Hovenia dulcis]]'' has been used in traditional [[Kampo|Japanese]], [[Traditional chinese medicine|Chinese]], and [[Traditional Korean medicine|Korean]] medicines to treat fever, parasitic infection, as a laxative, and a treatment of liver diseases, and as a [[hangover]] treatment.<ref name=Hyun>{{cite journal|last1=Hyun|first1=Tae|last2=Eom|first2=Seung|last3=Yu|first3=Chang|last4=Roitsch|first4=Thomas|title=Hovenia dulcis– An Asian Traditional Herb|journal=Planta Medica|volume=76|issue=10|year=2010|pages=943–949|issn=0032-0943|doi=10.1055/s-0030-1249776|pmid=20379955}}</ref> Methods have been developed to extract ampelopsin from it at large scales, and laboratory research has been conducted with the compound to see if it might be useful as a drug in any of the conditions for which the parent plant has been traditionally used.<ref name=Hyun /> |
||
In a trial of sixty patients with [[fatty liver disease]] dihydromyricetin improved glucose and lipid metabolism and exerted anti-inflammatory effects which were beneficial.<ref name="ChenZhao2015">{{cite journal|last1=Chen|first1=Shihui|last2=Zhao|first2=Xiaolan|last3=Wan|first3=Jing|last4=Ran|first4=Li|last5=Qin|first5=Yu|last6=Wang|first6=Xiaofang|last7=Gao|first7=Yanxiang|last8=Shu|first8=Furong|last9=Zhang|first9=Yong|last10=Liu|first10=Peng|last11=Zhang|first11=Qianyong|last12=Zhu|first12=Jundong|last13=Mi|first13=Mantian|title=Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial|journal=Pharmacological Research|volume=99|year=2015|pages=74–81|issn=10436618|doi=10.1016/j.phrs.2015.05.009}}</ref> |
In a trial of sixty patients with [[fatty liver disease]] dihydromyricetin improved glucose and lipid metabolism and exerted anti-inflammatory effects which were beneficial.<ref name="ChenZhao2015">{{cite journal|last1=Chen|first1=Shihui|last2=Zhao|first2=Xiaolan|last3=Wan|first3=Jing|last4=Ran|first4=Li|last5=Qin|first5=Yu|last6=Wang|first6=Xiaofang|last7=Gao|first7=Yanxiang|last8=Shu|first8=Furong|last9=Zhang|first9=Yong|last10=Liu|first10=Peng|last11=Zhang|first11=Qianyong|last12=Zhu|first12=Jundong|last13=Mi|first13=Mantian|title=Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial|journal=Pharmacological Research|volume=99|year=2015|pages=74–81|issn=10436618|doi=10.1016/j.phrs.2015.05.009}}</ref> |
||
Revision as of 13:42, 14 January 2016
| |
| Names | |
|---|---|
| IUPAC name
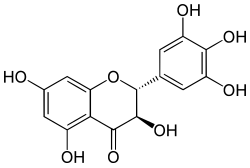
(2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one
| |
| Other names
Dihydromyricetin, Ampeloptin,(+)-Ampelopsin,(+)-Dihydromyricetin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H12O8 | |
| Molar mass | 320.253 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ampelopsin, also known as dihydromyricetin, is a flavanonol, a type of flavonoid. It is found in the Ampelopsis species japonica, megalophylla, and grossedentata; Cercidiphyllum japonicum; Hovenia dulcis; Rhododendron cinnabarinum; some Pinus species; and some Cedrus species,[1] as well as in Salix sachalinensis.[2]
Hovenia dulcis has been used in traditional Japanese, Chinese, and Korean medicines to treat fever, parasitic infection, as a laxative, and a treatment of liver diseases, and as a hangover treatment.[3] Methods have been developed to extract ampelopsin from it at large scales, and laboratory research has been conducted with the compound to see if it might be useful as a drug in any of the conditions for which the parent plant has been traditionally used.[3]
In a trial of sixty patients with fatty liver disease dihydromyricetin improved glucose and lipid metabolism and exerted anti-inflammatory effects which were beneficial.[4]
References
- ^ Zhou, Jiaju; Xie, Guirong; Yan, Xinjian (2011-02-21). Encyclopedia of Traditional Chinese Medicines – Molecular Structures, Pharmacological Activities, Natural Sources and Applications: Vol. 1: Isolated Compounds A-C. Springer Science & Business Media. ISBN 978-3-642-16735-5.
- ^ Tahara, Satoshi (June 23, 2007). "A Journey of Twenty-Five Years through the Ecological Biochemistry of Flavonoids". Bioscience, Biotechnology, and Biochemistry. 71 (6): 1387–1404. doi:10.1271/bbb.70028. ISSN 0916-8451. PMID 17587669.
- ^ a b Hyun, Tae; Eom, Seung; Yu, Chang; Roitsch, Thomas (2010). "Hovenia dulcis– An Asian Traditional Herb". Planta Medica. 76 (10): 943–949. doi:10.1055/s-0030-1249776. ISSN 0032-0943. PMID 20379955.
- ^ Chen, Shihui; Zhao, Xiaolan; Wan, Jing; Ran, Li; Qin, Yu; Wang, Xiaofang; Gao, Yanxiang; Shu, Furong; Zhang, Yong; Liu, Peng; Zhang, Qianyong; Zhu, Jundong; Mi, Mantian (2015). "Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial". Pharmacological Research. 99: 74–81. doi:10.1016/j.phrs.2015.05.009. ISSN 1043-6618.