Helpful Pixie Bot (talk | contribs) m ISBNs (Build KC) |
Maxim Masiutin (talk | contribs) Removed parameters. |
||
| (39 intermediate revisions by 30 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| Watchedfields = changed |
|||
| verifiedrevid = 443385694 |
|||
| Name = Ammonium hexachloroplatinate |
| verifiedrevid = 443387206 |
||
| Name = Ammonium hexachloroplatinate |
|||
| |
| ImageFile = (NH4)2PtCl6.svg |
||
| |
| ImageSize = 200px |
||
| |
| ImageName = Ammonium hexachloroplatinate |
||
| ImageFile2 = (NH4)2PtCl6Xray.tif |
|||
| ⚫ | |||
| ImageSize2 = 320px |
|||
| ⚫ | |||
| ImageName2 = Ammonium hexachloroplatinate |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ChemSpiderID = 10628022 |
| ChemSpiderID = 10628022 |
||
| PubChem = 16211460 |
|||
| EINECS = 240-973-0 |
|||
| InChI = 1/6ClH.2H3N.Pt/h6*1H;2*1H3;/q;;;;;;;;+4/p-4/rCl6Pt.2H3N/c1-7(2,3,4,5)6;;/h;2*1H3/q-2;;/p+2 |
| InChI = 1/6ClH.2H3N.Pt/h6*1H;2*1H3;/q;;;;;;;;+4/p-4/rCl6Pt.2H3N/c1-7(2,3,4,5)6;;/h;2*1H3/q-2;;/p+2 |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| Line 19: | Line 25: | ||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = PCCGQTHFYHJATL-UHFFFAOYSA-J |
| StdInChIKey = PCCGQTHFYHJATL-UHFFFAOYSA-J |
||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| CASNo = 16919-58-7 |
| CASNo = 16919-58-7 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 1653N9XMIC |
|||
}} |
}} |
||
| |
|Section2={{Chembox Properties |
||
| |
| Formula = (NH<sub>4</sub>)<sub>2</sub>PtCl<sub>6</sub> |
||
| |
| MolarMass = 443.87 g/mol |
||
| Odor = odorless |
|||
| ⚫ | |||
| Appearance = yellow crystals |
|||
| MeltingPt = |
|||
| ⚫ | |||
| Solvent = other solvents |
|||
| MeltingPtC = 380 |
|||
| SolubleOther = 0.5 g/100 mL (20 °C)<br />3.365 g/100 mL (100 °C) |
|||
| MeltingPt_notes = decomposes |
|||
| ⚫ | |||
| Solubility = 0.289 g/100ml (0 °C)<br /> 0.7 g/100ml (15 °C)<ref>{{cite web|url=http://chemister.ru/Database/properties-en.php?dbid=1&id=7145 |title=ammonium hexachloroplatinate(IV) |publisher=Chemister.ru |date=2007-03-19 |accessdate=2014-06-03}}</ref><br /> 0.499 g/100ml (20 °C)<br /> 3.36 g/100ml (100 °C)}} |
|||
| Section7 = {{Chembox Hazards |
|||
| Hazards_ref = |
|||
| ExternalSDS = |
|||
| GHSPictograms = {{GHS05}}{{GHS06}}{{GHS07}}{{GHS08}} |
|||
| GHSSignalWord = Danger |
|||
| HPhrases = {{H-phrases|290|301|317|318|334}} |
|||
| PPhrases = {{P-phrases|234|261|264|270|272|280|285|301+310|302+352|304+341|305+351+338|310|321|330|333+313|342+311|363|390|404|405|501}} |
|||
| MainHazards = |
|||
| IngestionHazard = |
|||
| InhalationHazard = |
|||
| EyeHazard = |
|||
| SkinHazard = |
|||
| NFPA-F = |
|||
| NFPA-H = |
|||
| NFPA-R = |
|||
| NFPA-S = |
|||
| NFPA_ref = |
|||
| FlashPt = |
|||
| FlashPtC = |
|||
| FlashPt_notes = |
|||
| FlashPt_ref = |
|||
| AutoignitionPt = |
|||
| AutoignitionPtC = |
|||
| AutoignitionPt_ref= |
|||
| AutoignitionPt_notes= |
|||
| ExploLimits = |
|||
| TLV = |
|||
| TLV-TWA = |
|||
| TLV-STEL = |
|||
| TLV-C = |
|||
| LD50 = 195 mg/kg rat |
|||
| LDLo = |
|||
| LC50 = |
|||
| LCLo = |
|||
| PEL = |
|||
| REL = |
|||
| IDLH = |
|||
| NIOSH_id = |
|||
| NIOSH_ref = |
|||
| ⚫ | |||
}} |
}} |
||
'''Ammonium hexachloroplatinate''', also known as ammonium chloroplatinate, is |
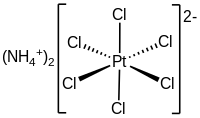
'''Ammonium hexachloroplatinate''', also known as ammonium chloroplatinate, is the [[inorganic compound]] with the formula (NH<sub>4</sub>)<sub>2</sub>[PtCl<sub>6</sub>]. It is a rare example of a soluble [[platinum]](IV) [[salt (chemistry)|salt]] that is not [[hygroscopy|hygroscopic]]. It forms intensely yellow solutions in water. In the presence of 1M [[ammonium chloride|NH<sub>4</sub>Cl]], its solubility is only 0.0028 g/100 mL. |
||
==Preparation and structure== |
==Preparation and structure== |
||
The compound consists of separate [[tetrahedral molecular geometry|tetrahedral]] [[ammonium]] |
The compound consists of separate [[tetrahedral molecular geometry|tetrahedral]] [[ammonium]] [[cation]]s and [[octahedral molecular geometry|octahedral]] [PtCl<sub>6</sub>]<sup>2−</sup> [[anion]]s. It is usually generated as a fine yellow precipitate by treating a solution of [[chloroplatinic acid|hexachloroplatinic acid]] with a solution of an ammonium salt.<ref name=Kauuf>{{cite book | chapter = Ammonium Hexachloroplatinate(IV) | author = George B. Kauffman | title = Inorganic Syntheses | author-link = George B. Kauffman | year = 1967 | volume = 9 | pages = 182–185 | doi = 10.1002/9780470132401.ch51 | isbn = 978-0-470-13240-1}}</ref> The complex is so poorly soluble that this step is employed in the isolation of platinum from ores and recycled residues.<ref>Cotton, S. A. ''Chemistry of Precious Metals'', Chapman and Hall (London): 1997. {{ISBN|0-7514-0413-6}}.</ref> |
||
As analyzed by [[X-ray crystallography]], the salt crystallizes in a cubic motif reminiscent of the [[calcium fluoride|fluorite]] structure. The [PtCl<sub>6</sub>]<sup>2−</sup> centers are octahedral. The NH<sub>4</sub><sup>+</sup> centers are [[hydrogen bond]]ed to the [[chloride]] [[ligands]].<ref>Verde-Gómez, Y.; Alonso-Nuñez, G.; Cervantes, F.; Keer, A. "Aqueous solution reaction to synthesize ammonium hexachloroplatinate and its crystallographic and thermogravimetric characterization" Materials Letters, 2003, volume 57, p 4667-4672. {{doi|10.1016/S0167-577X(03)00381-1}}</ref> |
|||
==Uses and reactions== |
==Uses and reactions== |
||
Ammonium hexachloroplatinate is used in platinum plating. |
Ammonium hexachloroplatinate is used in platinum plating. Heating (NH<sub>4</sub>)<sub>2</sub>[PtCl<sub>6</sub>] under a stream of [[hydrogen]] at 200 °C produces [[platinum]] sponge. Treating this with chlorine gives H<sub>2</sub>[PtCl<sub>6</sub>].<ref name=Kauuf/> |
||
Ammonium hexachloroplatinate decomposes to yield platinum sponge when heated to high temperatures:<ref name=Kauuf/><ref>{{cite book|title=Modern Descriptive Chemistry|last1=Rochow|first1=Eugene George|year=1977|publisher=W. B. Saunders Company|page=202|isbn=9780721676289|url=https://archive.org/details/moderndescriptiv0000roch/mode/1up}}</ref> |
|||
Heating [NH<sub>4</sub>]<sub>2</sub>[PtCl<sub>6</sub>] under a stream of [[hydrogen]] at 200 °C produces [[platinum]] sponge. Treating this with chlorine gives H<sub>2</sub>PtCl<sub>6</sub>.<ref name=Kauuf/> |
|||
:3(NH<sub>4</sub>)<sub>2</sub>PtCl<sub>6</sub> → 3Pt(s) + 2NH<sub>4</sub>Cl(g) + 16HCl(g) + 2N<sub>2</sub>(g) |
|||
==Safety== |
|||
Dust containing ammonium hexachloroplatinate can be highly allergenic. "Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma."<ref>{{cite book |doi=10.1002/14356007.a21_075|chapter=Platinum Group Metals and Compounds |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2001 |last1=Renner |first1=Hermann |last2=Schlamp |first2=Günther |last3=Kleinwächter |first3=Ingo |last4=Drost |first4=Ernst |last5=Lüschow |first5=Hans Martin |last6=Tews |first6=Peter |last7=Panster |first7=Peter |last8=Diehl |first8=Manfred |last9=Lang |first9=Jutta |last10=Kreuzer |first10=Thomas |last11=Knödler |first11=Alfons |last12=Starz |first12=Karl Anton |last13=Dermann |first13=Klaus |last14=Rothaut |first14=Josef |last15=Drieselmann |first15=Ralf |last16=Peter |first16=Catrin |last17=Schiele |first17=Rainer |isbn=3527306730 }}</ref> |
|||
==Related compounds== |
|||
*[[Potassium hexachloroplatinate]] |
|||
==References== |
==References== |
||
{{ |
{{Reflist}} |
||
{{Ammonium salts}} |
|||
| ⚫ | |||
{{Platinum compounds}} |
|||
| ⚫ | |||
[[fa:آمونیوم هگزاکلروپلاتینات]] |
|||
[[Category:Chloro complexes]] |
|||
[[ja:ヘキサクロロ白金(IV)酸アンモニウム]] |
|||
[[Category:Ammonium compounds]] |
|||
[[fi:Ammoniumheksakloroplatinaatti]] |
|||
[[Category:Hexachloroplatinates]] |
|||
Latest revision as of 13:25, 20 January 2024
| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium hexachloroplatinate(IV)
| |
| Other names
ammonium chloroplatinate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.233 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| (NH4)2PtCl6 | |
| Molar mass | 443.87 g/mol |
| Appearance | yellow crystals |
| Odor | odorless |
| Density | 3.065 g/cm3 |
| Melting point | 380 °C (716 °F; 653 K) decomposes |
| 0.289 g/100ml (0 °C) 0.7 g/100ml (15 °C)[1] 0.499 g/100ml (20 °C) 3.36 g/100ml (100 °C) | |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H290, H301, H317, H318, H334 | |
| P234, P261, P264, P270, P272, P280, P285, P301+P310, P302+P352, P304+P341, P305+P351+P338, P310, P321, P330, P333+P313, P342+P311, P363, P390, P404, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
195 mg/kg rat |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2[PtCl6]. It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. In the presence of 1M NH4Cl, its solubility is only 0.0028 g/100 mL.
Preparation and structure[edit]
The compound consists of separate tetrahedral ammonium cations and octahedral [PtCl6]2− anions. It is usually generated as a fine yellow precipitate by treating a solution of hexachloroplatinic acid with a solution of an ammonium salt.[2] The complex is so poorly soluble that this step is employed in the isolation of platinum from ores and recycled residues.[3]
As analyzed by X-ray crystallography, the salt crystallizes in a cubic motif reminiscent of the fluorite structure. The [PtCl6]2− centers are octahedral. The NH4+ centers are hydrogen bonded to the chloride ligands.[4]
Uses and reactions[edit]
Ammonium hexachloroplatinate is used in platinum plating. Heating (NH4)2[PtCl6] under a stream of hydrogen at 200 °C produces platinum sponge. Treating this with chlorine gives H2[PtCl6].[2]
Ammonium hexachloroplatinate decomposes to yield platinum sponge when heated to high temperatures:[2][5]
- 3(NH4)2PtCl6 → 3Pt(s) + 2NH4Cl(g) + 16HCl(g) + 2N2(g)
Safety[edit]
Dust containing ammonium hexachloroplatinate can be highly allergenic. "Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma."[6]
Related compounds[edit]
References[edit]
- ^ "ammonium hexachloroplatinate(IV)". Chemister.ru. 2007-03-19. Retrieved 2014-06-03.
- ^ a b c George B. Kauffman (1967). "Ammonium Hexachloroplatinate(IV)". Inorganic Syntheses. Vol. 9. pp. 182–185. doi:10.1002/9780470132401.ch51. ISBN 978-0-470-13240-1.
- ^ Cotton, S. A. Chemistry of Precious Metals, Chapman and Hall (London): 1997. ISBN 0-7514-0413-6.
- ^ Verde-Gómez, Y.; Alonso-Nuñez, G.; Cervantes, F.; Keer, A. "Aqueous solution reaction to synthesize ammonium hexachloroplatinate and its crystallographic and thermogravimetric characterization" Materials Letters, 2003, volume 57, p 4667-4672. doi:10.1016/S0167-577X(03)00381-1
- ^ Rochow, Eugene George (1977). Modern Descriptive Chemistry. W. B. Saunders Company. p. 202. ISBN 9780721676289.
- ^ Renner, Hermann; Schlamp, Günther; Kleinwächter, Ingo; Drost, Ernst; Lüschow, Hans Martin; Tews, Peter; Panster, Peter; Diehl, Manfred; Lang, Jutta; Kreuzer, Thomas; Knödler, Alfons; Starz, Karl Anton; Dermann, Klaus; Rothaut, Josef; Drieselmann, Ralf; Peter, Catrin; Schiele, Rainer (2001). "Platinum Group Metals and Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_075. ISBN 3527306730.