Content deleted Content added
حسن علي البط (talk | contribs) m Adding category Category:Phsnols (using HotCat) |
No edit summary |
||
| (36 intermediate revisions by 21 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| Watchedfields = changed |
|||
| ⚫ | |||
| verifiedrevid = 443958925 |
|||
| ImageSize = 200px |
|||
| ⚫ | |||
| ⚫ | |||
| ImageSizeL1 = 120px |
|||
| OtherNames = Ammelin, s-triazin-2-ol, 2,4-diamino-1,3,5-triazin-6-one |
|||
| ImageNameL1 = Structural formula |
|||
| ⚫ | |||
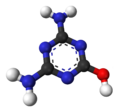
| ImageFileR1 = Ammeline-3D-balls.png |
|||
| ⚫ | |||
| ImageNameR1 = Ball-and-stick model |
|||
| ImageSizeR1 = 120px |
|||
| ⚫ | |||
| OtherNames = 2,4-Diamino-6-hydroxy-1,3,5-triazine<br />4,6-Diamino-2-hydroxy-1,3,5-triazine<br />4,6-diamino-1,3,5-triazin-2(1''H'')-one |
|||
| ⚫ | |||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 12063 |
|||
| InChIKey = MASBWURJQFFLOO-UHFFFAOYAL |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C3H5N5O/c4-1-6-2(5)8-3(9)7-1/h(H5,4,5,6,7,8,9) |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = MASBWURJQFFLOO-UHFFFAOYSA-N |
|||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| CASNo = 645-92-1 |
| CASNo = 645-92-1 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
| UNII = LC8G9556Q0 |
|||
| ⚫ | |||
| PubChem = 12583 |
| PubChem = 12583 |
||
| SMILES = |
| SMILES = O=C\1/N=C(/N)NC(=N/1)/N |
||
| InChI = 1/C3H5N5O/c4-1-6-2(5)8-3(9)7-1/h(H5,4,5,6,7,8,9) |
|||
| InChI = |
|||
| RTECS = |
| RTECS = |
||
| MeSHName = |
| MeSHName = |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = |
|||
| |
| ChEBI = 28646 |
||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| ATCCode_prefix = |
|||
| KEGG = C08733 |
|||
| ATCCode_suffix = |
|||
}} |
|||
| ATC_Supplemental =}} |
|||
| |
|Section2={{Chembox Properties |
||
| C=3 | H=5 | N=5 | O=1 |
|||
| Formula = C<sub>3</sub>H<sub>5</sub>N<sub>5</sub>O |
|||
| MolarMass = 127.11 g/mol |
|||
| Appearance = White powder |
| Appearance = White powder |
||
| Density = |
| Density = |
||
| MeltingPt = N/A |
| MeltingPt = N/A |
||
| |
| MeltingPt_notes = (decomposes before melting) |
||
| BoilingPt = |
| BoilingPt = |
||
| BoilingPt_notes = |
|||
| Boiling_notes = |
|||
| Solubility = |
| Solubility = Trace |
||
| SolubleOther = |
| SolubleOther = Soluble in aqueous alkalies and mineral acids, but not [[acetic acid]] |
||
| Solvent = |
| Solvent = |
||
| pKa = |
| pKa = |
||
| pKb = }} |
| pKb = }} |
||
| |
|Section7={{Chembox Hazards |
||
| |
| MainHazards = |
||
| |
| NFPA-H = |
||
| |
| NFPA-F = |
||
| NFPA- |
| NFPA-R = |
||
| NFPA- |
| NFPA-S = |
||
| |
| FlashPt = |
||
| |
| AutoignitionPt = |
||
| |
| ExploLimits = |
||
| SPhrases = |
|||
| RSPhrases = |
|||
| FlashPt = |
|||
| Autoignition = |
|||
| ExploLimits = |
|||
| PEL = }} |
| PEL = }} |
||
}} |
}} |
||
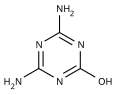
'''Ammeline''' (4,6- |
'''Ammeline''' ('''4,6-diamino-2-hydroxy-1,3,5-triazine''') is a triazine [[derivative (chemistry)|derivative]]. It is the hydrolysis product of [[melamine]].<ref>{{cite journal | author = B. Bann and S.A. Miller | title = Melamines and derivatives of melamine | journal = [[Chemical Reviews]] | doi = 10.1021/cr50019a004 | volume = 58 | pages = 131–172 | date = 1958}}</ref> |
||
==Synthesis== |
==Synthesis== |
||
Ammeline can be synthesized by the pyrolysis of urea |
Ammeline can be synthesized by the [[pyrolysis]] of [[urea]] or the [[condensation reaction]] among 2 moles of [[dicyandiamide]] and 1 mole of [[biuret]]. |
||
: |
: 2 C<sub>2</sub>H<sub>4</sub>N<sub>4</sub> + C<sub>2</sub>H<sub>5</sub>N<sub>3</sub>O<sub>2</sub> → 2C<sub>3</sub>H<sub>5</sub>N<sub>5</sub>O + NH<sub>3</sub> |
||
==Chemical |
==Chemical properties== |
||
Ammeline is weakly acidic with [[pKa]] ~9. It can form [[nitrate]], [[sulfate]], [[chromate]] and oxalate salts. Ammeline reacts with boiling dilute [[hydrochloric acid]] to form [[melem]] and [[ammonia]]. |
Ammeline is weakly acidic with [[pKa]] ~9. It can form [[nitrate]], [[sulfate]], [[Monochromate|chromate]], and [[oxalate]] [[salt (chemistry)|salts]]. Ammeline reacts with boiling dilute [[hydrochloric acid]] to form [[melem]] and [[ammonia]]. |
||
Ammeline is the first step in melamine hydrolysis. Further hydrolysis (e.g. boiling ammeline with dilute alkali) yields [[ammelide]]. |
Ammeline is the first step in melamine hydrolysis. Further hydrolysis (e.g. boiling ammeline with dilute alkali) yields [[ammelide]]. |
||
==References== |
==References== |
||
{{reflist}} |
|||
* B. Bann and S.A. Miller, "Melamines and derivatives of melamine", [[Chemical Reviews]], vol.58, p131-172 (1958). |
|||
[[Category:Triazines]] |
[[Category:Triazines]] |
||
[[Category: |
[[Category:Aromatic amines]] |
||
Latest revision as of 14:53, 5 January 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4,6-Diamino-1,3,5-triazin-2-ol | |||
| Other names
2,4-Diamino-6-hydroxy-1,3,5-triazine
4,6-Diamino-2-hydroxy-1,3,5-triazine 4,6-diamino-1,3,5-triazin-2(1H)-one | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.415 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H5N5O | |||
| Molar mass | 127.107 g·mol−1 | ||
| Appearance | White powder | ||
| Melting point | N/A (decomposes before melting) | ||
| Trace | |||
| Solubility | Soluble in aqueous alkalies and mineral acids, but not acetic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ammeline (4,6-diamino-2-hydroxy-1,3,5-triazine) is a triazine derivative. It is the hydrolysis product of melamine.[1]
Synthesis[edit]
Ammeline can be synthesized by the pyrolysis of urea or the condensation reaction among 2 moles of dicyandiamide and 1 mole of biuret.
- 2 C2H4N4 + C2H5N3O2 → 2C3H5N5O + NH3
Chemical properties[edit]
Ammeline is weakly acidic with pKa ~9. It can form nitrate, sulfate, chromate, and oxalate salts. Ammeline reacts with boiling dilute hydrochloric acid to form melem and ammonia.
Ammeline is the first step in melamine hydrolysis. Further hydrolysis (e.g. boiling ammeline with dilute alkali) yields ammelide.
References[edit]
- ^ B. Bann and S.A. Miller (1958). "Melamines and derivatives of melamine". Chemical Reviews. 58: 131–172. doi:10.1021/cr50019a004.