Content deleted Content added
حسن علي البط (talk | contribs) m Adding category Category:Phenols (using HotCat) |
|||
| Line 64: | Line 64: | ||
[[Category:Triazines]] |
[[Category:Triazines]] |
||
[[Category:Phenols]] |
|||
Revision as of 07:27, 10 April 2010
| |
| Names | |
|---|---|
| IUPAC name
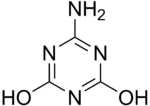
6-Amino-2,4-Dihydroxy-l,3,5-Triazine
| |
| Other names
Ammelid, 2-Amino-1,3,5-triazine-4,6-dione, 2-Amino-4,6-dihydroxy-s-triazine
| |
| Identifiers | |
| ECHA InfoCard | 100.010.416 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C3H4N4O2 | |
| Molar mass | 128.09 g/mol |
| Appearance | white powder |
| insoluble | |
| Solubility | soluble in concentrated mineral acids, alkalies and ammonia |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ammelide (6-Amino-2,4-Dihydroxy-l,3,5-Triazine) is a triazine and the hydrolysis product of ammeline.
Synthesis
Ammelide can be obtained by heating dicyandiamide with aqueous ammonia at 160-170°C. It can also be synthezised by heating melam with concentrated sulfuric acid for a short time at 190°C.
Chemical property
Ammelide forms salts with both acids (hydrochloric acid, nitric acid, sulfuric acid)and bases (sodium hydroxide, ammonium, calcium hydroxide).
Ammelide decomposes at 170oC with water to form carbon dioxide and ammonia. It can be converted into cyanuric acid by oxidizing agents (e.g. potassium permanganate) or by boiling with acids or alkalies.
References
- B. Bann and S.A. Miller, "Melamines and derivatives of melamine", Chemical Reviews, vol.58, p131-172 (1958).