Content deleted Content added
Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi |
Shinkolobwe (talk | contribs) Adding short description: "Organosulfur compound", overriding automatically generated description Tag: Shortdesc helper |
||
| (33 intermediate revisions by 27 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Organosulfur compound}} |
|||
{{chembox |
|||
{{Chembox |
|||
| ⚫ | |||
| Verifiedfields = changed |
|||
|Reference=<ref>[http://www.sigmaaldrich.com/catalog/ProductDetail.do?N4=A34201|ALDRICH&N5=SEARCH_CONCAT_PNO|BRAND_KEY&F=SPEC Allyl methyl sulfide] at [[Sigma-Aldrich]]</ref> |
|||
| Watchedfields = changed |
|||
|ImageFile=Allyl methyl sulfide.png |
|||
| ⚫ | |||
| ⚫ | |||
| ImageFile = Allylmethyl sulfide Structural Formula V1.svg |
|||
|IUPACName=3-Methylsulfanylprop-1-ene |
|||
| ImageFile_Ref = {{chemboximage|correct|??}} |
|||
| ⚫ | |||
| ⚫ | |||
| ImageName = XYZ |
|||
| PIN = 3-(Methylsulfanyl)prop-1-ene |
|||
| ⚫ | |||
3-Methylthio-1-propene |
|||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo_Ref = {{cascite|changed|??}} |
|||
| ⚫ | |||
| ⚫ | |||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| UNII = V7QI1R316C |
|||
| ⚫ | |||
| ChemSpiderID = 21159856 |
| ChemSpiderID = 21159856 |
||
| ⚫ | |||
| ⚫ | |||
| EINECS = 233-422-0 |
|||
| ⚫ | |||
| |
| UNNumber = 1993 |
||
| MeSHName = allyl+methyl+sulfide |
|||
| ⚫ | |||
| RTECS = UD1015000 |
|||
| ⚫ | |||
| StdInChI = 1S/C4H8S/c1-3-4-5-2/h3H,1,4H2,2H3 |
| StdInChI = 1S/C4H8S/c1-3-4-5-2/h3H,1,4H2,2H3 |
||
| |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| ⚫ | |||
| StdInChIKey = NVLPQIPTCCLBEU-UHFFFAOYSA-N |
| StdInChIKey = NVLPQIPTCCLBEU-UHFFFAOYSA-N |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| C=4 | H=8 | S=1 |
|||
| Formula=C<sub>4</sub>H<sub>8</sub>S |
|||
| Odor = Garlic |
|||
| MolarMass=88.17 g/mol |
|||
| Density = 0.803 g cm<sup>−3</sup> |
|||
| Appearance= |
|||
| BoilingPtK = 365 |
|||
| Density=0.803 g/mL |
|||
| ⚫ | |||
| MeltingPt= |
|||
| BoilingPt=91-93 °C |
|||
| Solubility= |
|||
| ⚫ | |||
|Section3={{Chembox Hazards |
|Section3={{Chembox Hazards |
||
| GHSPictograms = {{GHS flame}} |
|||
| MainHazards= |
|||
| GHSSignalWord = '''DANGER''' |
|||
| FlashPt=18 °C (65 °F) |
|||
| HPhrases = {{H-phrases|225}} |
|||
| Autoignition= |
|||
| |
| PPhrases = {{P-phrases|210}} |
||
| FlashPtC = 18.0 |
|||
| SPhrases={{S16}} {{S29}} {{S33}} |
|||
}} |
|||
}} |
}} |
||
'''Allyl methyl sulfide''' |
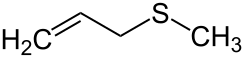
'''Allyl methyl sulfide''' is an [[organosulfur compound]] with the [[chemical formula]] CH<sub>2</sub>=CHCH<sub>2</sub>SCH<sub>3</sub>. The molecule features two [[functional group]]s, an [[allyl]] (CH<sub>2</sub>=CHCH<sub>2</sub>) and a [[thioether|sulfide]]. It is a colourless liquid with a strong odor characteristic of alkyl sulfides. It is a metabolite of [[garlic]], and "[[garlic breath]]" is attributed to its presence.<ref>{{cite book| author = Eric Block| title = Garlic and Other Alliums: The Lore and the Science| date = 2010-01-04| publisher = Royal Society of Chemistry| isbn = 978-0-85404-190-9 }}</ref> |
||
It is prepared by the reaction of [[allyl chloride]] with [[sodium hydroxide]] and [[methanethiol]]. |
|||
:CH<sub>2</sub>=CHCH<sub>2</sub>Cl + NaOH (aq) + CH<sub>3</sub>SH → CH<sub>2</sub>=CHCH<sub>2</sub>SCH<sub>3</sub> + NaCl + H<sub>2</sub>O |
|||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
[[Category:Thioethers]] |
[[Category:Thioethers]] |
||
[[Category: |
[[Category:Allyl compounds]] |
||
[[fr:Allyl méthyl thioéther]] |
|||
[[nl:Allylmethylsulfide]] |
|||
Latest revision as of 20:49, 2 August 2022
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-(Methylsulfanyl)prop-1-ene | |
| Other names
Methyl propenyl sulfide
3-Methylthio-1-propene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.371 |
| EC Number |
|
| MeSH | allyl+methyl+sulfide |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8S | |
| Molar mass | 88.17 g·mol−1 |
| Odor | Garlic |
| Density | 0.803 g cm−3 |
| Boiling point | 92 °C; 197 °F; 365 K |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H225 | |
| P210 | |
| Flash point | 18.0 °C (64.4 °F; 291.1 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Allyl methyl sulfide is an organosulfur compound with the chemical formula CH2=CHCH2SCH3. The molecule features two functional groups, an allyl (CH2=CHCH2) and a sulfide. It is a colourless liquid with a strong odor characteristic of alkyl sulfides. It is a metabolite of garlic, and "garlic breath" is attributed to its presence.[1]
It is prepared by the reaction of allyl chloride with sodium hydroxide and methanethiol.
- CH2=CHCH2Cl + NaOH (aq) + CH3SH → CH2=CHCH2SCH3 + NaCl + H2O