حسن علي البط (talk | contribs) added Category:Hydroxyquinones using HotCat |
Citation bot (talk | contribs) Add: pmc, pmid, pages, issue, volume, journal, date, title, authors 1-1. | Use this bot. Report bugs. | Suggested by Marbletan | #UCB_webform |
||
| (41 intermediate revisions by 26 users not shown) | |||
| Line 7: | Line 7: | ||
| ImageFile1 = Alkannin 3D spacefill.png |
| ImageFile1 = Alkannin 3D spacefill.png |
||
| ImageAlt1 = Space-filling model of the alkannin molecule |
| ImageAlt1 = Space-filling model of the alkannin molecule |
||
| |
| PIN = 5,8-Dihydroxy-2-[(1''S'')-1-hydroxy-4-methylpent-3-en-1-yl]naphthalene-1,4-dione |
||
| OtherNames = C.I. Natural red 20 |
| OtherNames = {{Unbulleted list|C.I. Natural red 20|Alkanet extract|Anchusaic acid|Anchusin}} |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| KEGG_Ref = {{keggcite|correct|kegg}} |
| KEGG_Ref = {{keggcite|correct|kegg}} |
||
| Line 19: | Line 19: | ||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = NEZONWMXZKDMKF-JTQLQIEISA-N |
| StdInChIKey = NEZONWMXZKDMKF-JTQLQIEISA-N |
||
| CASNo_Ref = {{cascite| |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo = 517-88-4 |
| CASNo = 517-88-4 |
||
| UNII_Ref = {{cascite|correct|CAS}} |
|||
| UNII = 075CRZ9995 |
|||
| PubChem = 72521 |
| PubChem = 72521 |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| Line 44: | Line 46: | ||
}} |
}} |
||
}} |
}} |
||
'''Alkannin''' is a [[natural dye]] that is obtained from the extracts of plants from the borage family ''[[Alkanna tinctoria]]'' that are found in the south of France. The dye is used as a [[food coloring]] and in cosmetics. It is used as a red-brown [[food additive]] in regions such as Australia,<ref>[http://www.foodstandards.gov.au/_srcfiles/Additives%20alpha.pdf Additives], [[Food Standards Australia New Zealand]]</ref> and is designated in Europe as the [[E number]] E103, but is no longer approved for use.<ref>"[http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist Current EU approved additives and their E Numbers]", Food Standards Agency website, retrieved 15 Dec 2011</ref> Alkannin has a deep red color in a greasy or oily environment and a violet color in an alkaline environment.{{cn|date=May 2017}} |
|||
The chemical structure as a [[naphthoquinone]] derivative was first determined by Brockmann in 1936.<ref>{{cite journal | doi = 10.1002/jlac.19365210102 | author = H. Brockmann | title = Die Konstitution des Alkannins, Shikonins und Alkannans | journal = Justus Liebigs Ann. Chem. | year = 1936 | volume = 521 | pages = 1–47}}</ref> |
'''Alkannin''' is a [[natural dye]] that is obtained from the extracts of ''[[Alkanna tinctoria]]'' which is found in the south of France. The dye is used as a [[food coloring|food coloring]] and in cosmetics; the European [[E number]] schedule, it is numbered '''E103'''. It is used as a red-brown [[food additive]] in regions such as Australia.<ref>[http://www.foodstandards.gov.au/_srcfiles/Additives%20alpha.pdf Additives] {{Webarchive|url=https://web.archive.org/web/20110406040011/http://www.foodstandards.gov.au/_srcfiles/Additives%20alpha.pdf |date=2011-04-06 }}, [[Food Standards Australia New Zealand]]</ref> Alkannin is deep red in an acid and blue in an alkaline environment.<ref>"Alkanet" in [http://www.henriettesherbal.com/eclectic/usdisp/alkanna.html ''Dispensatory of the United States of America'', year 1918], edited by Joseph P. Remington and Horatio C. Wood.</ref> The chemical structure as a [[naphthoquinone]] derivative was first determined by Brockmann in 1936.<ref>{{cite journal | doi = 10.1002/jlac.19365210102 | author = H. Brockmann | title = Die Konstitution des Alkannins, Shikonins und Alkannans | journal = Justus Liebigs Ann. Chem. | year = 1936 | volume = 521 | pages = 1–47}}</ref> The ''R''-[[enantiomer]] of alkannin is known as '''shikonin''', and the [[racemic mixture]] of the two is known as '''shikalkin'''.<ref>{{cite book | title = Dictionary of Food Compounds | page = 478 | author = Shmuel Yannai | publisher = CRC Press | date = 2012}}</ref><ref name=Nicolaou>{{cite journal | doi = 10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0 | title = The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products | author = Vassilios P. Papageorgiou |author2=Andreana N. Assimopoulou |author3=Elias A. Couladouros |author4=David Hepworth | author5-link = K. C. Nicolaou |author5=K. C. Nicolaou |display-authors=3| journal = Angew. Chem. Int. Ed. | year = 1999 | volume = 38 | pages = 270–300 | issue = 3| pmid = 29711637 }}</ref> |
||
== Biosynthesis == |
|||
The enzyme [[4-hydroxybenzoate geranyltransferase]] utilizes [[Geranyl pyrophosphate|geranyl diphosphate]] and [[4-Hydroxybenzoic acid|4-hydroxybenzoate]] to produce [[3-geranyl-4-hydroxybenzoate]] and diphosphate. These compounds are then used to form alkannin.<ref name=Nicolaou/> |
The enzyme [[4-hydroxybenzoate geranyltransferase]] utilizes [[Geranyl pyrophosphate|geranyl diphosphate]] and [[4-Hydroxybenzoic acid|4-hydroxybenzoate]] to produce [[3-geranyl-4-hydroxybenzoate]] and diphosphate. These compounds are then used to form alkannin.<ref name="Nicolaou" /> |
||
==Research == |
|||
Alkannin is an antioxidant<ref>{{cite journal | doi = 10.1016/j.foodchem.2003.12.017 | title = Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates |author1=A.N. Assimopoulou |author2=D. Boskou |author3=V.P. Papageorgiou | journal = Food Chemistry | volume = 87 | year = 2004 | pages = 433–438 | issue = 3}}</ref> and has an antimicrobial effect against ''[[Staphylococcus aureus]]'' and ''[[Staphylococcus epidermidis]]''. It is also known to have wound healing, antitumor, and antithrombotic properties.<ref name=Nicolaou/> |
|||
Because the root bark (cork layers) of ''Alkanna tinctoria'' contains large amounts of red [[naphthoquinone]] pigments, including alkannin, the roots of these plants are red-purple. When extracted from fresh tissues, the pigment gradually darkens over several days, finally forming black precipitates, which are thought to be polymers.<ref name="Kazufumi">{{cite journal | doi = 10.5511/plantbiotechnology.17.0823a | title = ''Lithospermum erythrorhizon'' cell cultures: Present and future aspects | date = 2017 | last1 = Yazaki | first1 = Kazufumi | journal = Plant Biotechnology | volume = 34 | issue = 3 | pages = 131–142 | pmid = 31275019 | pmc = 6565996 }}</ref> |
|||
[[File:Shikonin-Directly-Targets-Mitochondria-and-Causes-Mitochondrial-Dysfunction-in-Cancer-Cells-726025.f2.ogv|thumb|right|Effect of shikonin on the microtubule cytoskeleton.<ref>{{cite journal | last1 = Wiench | first1 = B | last2 = Eichhorn | first2 = T | last3 = Paulsen | first3 = M | last4 = Efferth | first4 = T | year = 2012 | title = Shikonin Directly Targets Mitochondria and Causes Mitochondrial Dysfunction in Cancer Cells | url = | journal = Evidence-Based Complementary and Alternative Medicine | volume = 2012 | issue = | page = 726025 | doi = 10.1155/2012/726025 | pmid = 23118796 |pmc=3478753 }}</ref>]] |
|||
Shikonin is also found in the Chinese herbal medicine plant ''[[Lithospermum erythrorhizon]]'', the red-root gromwell, (紫草 ''zicao'', [[Pinyin]]: zǐcǎo). The dried root is a Chinese herbal medicine with various antiviral and biological activities, including inhibition of human immunodeficiency virus type 1 (HIV-1).<ref>{{cite journal | last1=Chen | first1=X| pmid = 12936978 | volume=47 | issue=9 | title=Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. | date=Sep 2003 | journal=Antimicrob Agents Chemother | pages=2810–6 | doi=10.1128/aac.47.9.2810-2816.2003 | pmc=182643}}</ref><ref>{{cite journal | last1 = Gao | first1 = H. | year = 2011 | title = Anti-adenovirus activities of shikonin, a component of Chinese herbal medicine in vitro | url = | journal = Biol Pharm Bull | volume = 34 | issue = 2| pages = 197–202 | doi=10.1248/bpb.34.197|display-authors=etal}}</ref><ref>{{cite journal | last1 = Chen | first1 = J | last2 = Xie | first2 = J | last3 = Jiang | first3 = Z | last4 = Wang | first4 = B | last5 = Wang | first5 = Y | last6 = Hu | first6 = X | year = 2011 | title = Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2 | url = | journal = Oncogene | volume = 30 | issue = | pages = 4297–4306 | doi = 10.1038/onc.2011.137 | pmid=21516121}}</ref> |
|||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
== External links == |
|||
<!-- {{commons}} --> |
|||
* [http://www.sigmaaldrich.com/catalog/product/sigma/s7576?lang=fr®ion=FR Shikonin at Sigma-Aldrich] |
|||
[[Category:Food colorings]] |
[[Category:Food colorings]] |
||
[[Category:1,4-Naphthoquinones]] |
[[Category:1,4-Naphthoquinones]] |
||
| ⚫ | |||
[[Category:Terpeno-phenolic compounds]] |
[[Category:Terpeno-phenolic compounds]] |
||
[[Category:Articles containing video clips]] |
[[Category:Articles containing video clips]] |
||
[[Category: |
[[Category:Hydroxynaphthoquinones]] |
||
| ⚫ | |||
[[Category:3-Hydroxypropenals within hydroxyquinones]] |
|||
[[Category:Alkene derivatives]] |
|||
Revision as of 19:07, 19 March 2024
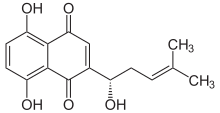
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
5,8-Dihydroxy-2-[(1S)-1-hydroxy-4-methylpent-3-en-1-yl]naphthalene-1,4-dione | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.497 |
| E number | E103 (colours) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C16H16O5 | |
| Molar mass | 288.299 g·mol−1 |
| Appearance | Red-brown crystalline prisms |
| Density | 1.15 g/mL |
| Melting point | 149 °C (300 °F; 422 K) |
| Boiling point | 567 °C (1,053 °F; 840 K) |
| Sparingly soluble | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3.0 g/kg (mice) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Alkannin is a natural dye that is obtained from the extracts of Alkanna tinctoria which is found in the south of France. The dye is used as a food coloring and in cosmetics; the European E number schedule, it is numbered E103. It is used as a red-brown food additive in regions such as Australia.[2] Alkannin is deep red in an acid and blue in an alkaline environment.[3] The chemical structure as a naphthoquinone derivative was first determined by Brockmann in 1936.[4] The R-enantiomer of alkannin is known as shikonin, and the racemic mixture of the two is known as shikalkin.[5][6]
Biosynthesis
The enzyme 4-hydroxybenzoate geranyltransferase utilizes geranyl diphosphate and 4-hydroxybenzoate to produce 3-geranyl-4-hydroxybenzoate and diphosphate. These compounds are then used to form alkannin.[6]
Research
Because the root bark (cork layers) of Alkanna tinctoria contains large amounts of red naphthoquinone pigments, including alkannin, the roots of these plants are red-purple. When extracted from fresh tissues, the pigment gradually darkens over several days, finally forming black precipitates, which are thought to be polymers.[7]
References
- ^ The Merck Index, 11th Edition, 243
- ^ Additives Archived 2011-04-06 at the Wayback Machine, Food Standards Australia New Zealand
- ^ "Alkanet" in Dispensatory of the United States of America, year 1918, edited by Joseph P. Remington and Horatio C. Wood.
- ^ H. Brockmann (1936). "Die Konstitution des Alkannins, Shikonins und Alkannans". Justus Liebigs Ann. Chem. 521: 1–47. doi:10.1002/jlac.19365210102.
- ^ Shmuel Yannai (2012). Dictionary of Food Compounds. CRC Press. p. 478.
- ^ a b Vassilios P. Papageorgiou; Andreana N. Assimopoulou; Elias A. Couladouros; et al. (1999). "The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products". Angew. Chem. Int. Ed. 38 (3): 270–300. doi:10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. PMID 29711637.
- ^ Yazaki, Kazufumi (2017). "Lithospermum erythrorhizon cell cultures: Present and future aspects". Plant Biotechnology. 34 (3): 131–142. doi:10.5511/plantbiotechnology.17.0823a. PMC 6565996. PMID 31275019.