Content deleted Content added
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChemSpiderID InChI1 InChIKey1 SMILES1. |
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: InChI1->InChI StdInChI StdInChIKey. |
||
| Line 8: | Line 8: | ||
|Section1= {{Chembox Identifiers |
|Section1= {{Chembox Identifiers |
||
| ChemSpiderID = 4444274 |
| ChemSpiderID = 4444274 |
||
| |
| InChI = 1/C18H16O7/c1-22-10-7-12(20)15-14(8-10)25-17(18(24-3)16(15)21)9-4-5-13(23-2)11(19)6-9/h4-8,19-20H,1-3H3 |
||
| |
| InChIKey = KPCRYSMUMBNTCK-UHFFFAOYAE |
||
| SMILES1 = O=C1c3c(O/C(=C1/OC)c2ccc(OC)c(O)c2)cc(OC)cc3O |
| SMILES1 = O=C1c3c(O/C(=C1/OC)c2ccc(OC)c(O)c2)cc(OC)cc3O |
||
| StdInChI = 1S/C18H16O7/c1-22-10-7-12(20)15-14(8-10)25-17(18(24-3)16(15)21)9-4-5-13(23-2)11(19)6-9/h4-8,19-20H,1-3H3 |
|||
| StdInChIKey = KPCRYSMUMBNTCK-UHFFFAOYSA-N |
|||
| CASNo = 572-32-7 |
| CASNo = 572-32-7 |
||
| CASNo_Ref = |
| CASNo_Ref = |
||
Revision as of 16:05, 29 November 2010
| |
| Names | |
|---|---|
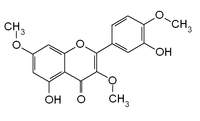
| IUPAC name
5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,7-dimethoxychromen-4-one
| |
| Other names
3,7,4'-Tri-O-methylquercetin
3,7,4'-trimethylquercetin 5,3'-dihydroxy-3,7,4'-trimethoxyflavone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H16O7 | |
| Molar mass | 344.31 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ayanin is a O-methylated flavonol, a type of flavonoid. It is the 3,7,4'-tri-O-methylation of quercetin.
It can be found in Croton schiedeanus. It can also be synthetized[1].
Biosynthetis
The enzyme 3,7-dimethylquercetin 4'-O-methyltransferase uses S-adenosyl methionine and 5,3',4'-trihydroxy-3,7-dimethoxyflavone (rhamnazin) to produce S-adenosylhomocysteine and 5,3'-dihydroxy-3,7,4'-trimethoxyflavone (ayanin).