Content deleted Content added
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: StdInChI StdInChIKey. |
Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi |
||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| verifiedrevid = 399533067 |
|||
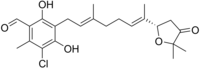
| Name = Ascofuranone |
| Name = Ascofuranone |
||
| ImageFile = Ascofuranone structure.png |
| ImageFile = Ascofuranone structure.png |
||
| Line 9: | Line 10: | ||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| SMILES = O=Cc1c(O)c(c(O)c(Cl)c1C)C/C=C(/CC/C=C(\C)[C@H]2OC(C(=O)C2)(C)C)C |
| SMILES = O=Cc1c(O)c(c(O)c(Cl)c1C)C/C=C(/CC/C=C(\C)[C@H]2OC(C(=O)C2)(C)C)C |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| |
| ChemSpiderID = 4939184 |
||
| PubChem = 6434242 |
| PubChem = 6434242 |
||
| InChI = 1/C23H29ClO5/c1-13(7-6-8-14(2)18-11-19(26)23(4,5)29-18)9-10-16-21(27)17(12-25)15(3)20(24)22(16)28/h8-9,12,18,27-28H,6-7,10-11H2,1-5H3/b13-9+,14-8+/t18-/m0/s1 |
| InChI = 1/C23H29ClO5/c1-13(7-6-8-14(2)18-11-19(26)23(4,5)29-18)9-10-16-21(27)17(12-25)15(3)20(24)22(16)28/h8-9,12,18,27-28H,6-7,10-11H2,1-5H3/b13-9+,14-8+/t18-/m0/s1 |
||
| InChIKey = VGYPZLGWVQQOST-JUERRSSIBJ |
| InChIKey = VGYPZLGWVQQOST-JUERRSSIBJ |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| |
| StdInChI = 1S/C23H29ClO5/c1-13(7-6-8-14(2)18-11-19(26)23(4,5)29-18)9-10-16-21(27)17(12-25)15(3)20(24)22(16)28/h8-9,12,18,27-28H,6-7,10-11H2,1-5H3/b13-9+,14-8+/t18-/m0/s1 |
||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = VGYPZLGWVQQOST-JUERRSSISA-N |
| StdInChIKey = VGYPZLGWVQQOST-JUERRSSISA-N |
||
| CASNo =38462-04-3 |
| CASNo =38462-04-3 |
||
Revision as of 16:02, 29 November 2010
| |
| Names | |
|---|---|
| IUPAC name
5-chloro-3-[(2E,6E)-7-[(2S)-5,5-dimethyl-4-oxo-tetrahydrofuran-2-yl]-3-methyl-octa-2,6-dienyl]-2,4-dihydroxy-6-methyl-benzaldehyde
| |
| Other names
Ascofuranon
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H29ClO5 | |
| Molar mass | 420.93 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ascofuranone is an antibiotic produced by the fungus Ascochyta visiae[1] that inhibits the Trypanosoma brucei alternative oxidase and is a lead compound in efforts to produce other drugs targeting this enzyme for the treatment of sleeping sickness.[2] The compound is effective both in vitro cell culture and in infections in mice.[1]
Ascofuranone has also been reported to have anti-tumor activity,[3] and modulate the immune system.[4]
References
- ^ a b Yabu Y, Yoshida A, Suzuki T, Nihei C, Kawai K, Minagawa N, Hosokawa T, Nagai K, Kita K, Ohta N (2003). "The efficacy of ascofuranone in a consecutive treatment on Trypanosoma brucei brucei in mice". Parasitol. Int. 52 (2): 155–64. doi:10.1016/S1383-5769(03)00012-6. PMID 12798927.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Minagawa N, Yabu Y, Kita K, Nagai K, Ohta N, Meguro K, Sakajo S, Yoshimoto A (1997). "An antibiotic, ascofuranone, specifically inhibits respiration and in vitro growth of long slender bloodstream forms of Trypanosoma brucei brucei". Mol. Biochem. Parasitol. 84 (2): 271–80. doi:10.1016/S0166-6851(96)02797-1. PMID 9084049.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Magae J, Hayasaki J, Matsuda Y, Hotta M, Hosokawa T, Suzuki S, Nagai K, Ando K, Tamura G (1988). "Antitumor and antimetastatic activity of an antibiotic, ascofuranone, and activation of phagocytes". J. Antibiot. 41 (7): 959–65. PMID 3417568.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Magae J, Suzuki S, Nagai K, Yamasaki M, Ando K, Tamura G (1986). "In vitro effects of an antitumor antibiotic, ascofuranone, on the murine immune system". Cancer Res. 46 (3): 1073–8. PMID 3080231.
{{cite journal}}: CS1 maint: multiple names: authors list (link)