Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChemSpiderID InChI InChIKey SMILES InChI1->InChI. |
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: StdInChI StdInChIKey. |
||
| Line 8: | Line 8: | ||
| InChIKey = CAMXVZOXBADHNJ-UHFFFAOYAU |
| InChIKey = CAMXVZOXBADHNJ-UHFFFAOYAU |
||
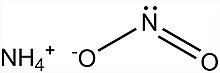
| SMILES = [O-]N=O.[NH4+] |
| SMILES = [O-]N=O.[NH4+] |
||
| StdInChI = 1S/HNO2.H3N/c2-1-3;/h(H,2,3);1H3 |
|||
| StdInChIKey = CAMXVZOXBADHNJ-UHFFFAOYSA-N |
|||
| CASNo = 13446-48-5 |
| CASNo = 13446-48-5 |
||
}} |
}} |
||
Revision as of 14:03, 29 November 2010
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.257 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NH4NO2 | |
| Molar mass | 64.06 g/mol |
| Appearance | pale yellow crystals, slowly decomposes to nitrogen and water |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
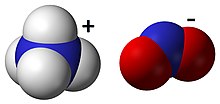
Ammonium nitrite, NH4NO2, is a salt containing ammonium and nitrite ions. It is used as a rodenticide, microbiocide and agricultural pesticide, and is acutely toxic to both humans and aquatic organisms.[1]
Preparation
Ammonium nitrite forms naturally in the air and can be prepared by the absorption of equal parts nitrogen dioxide and nitric oxide upon aqueous ammonia.[2]
It can also be prepared by oxidizing ammonia with ozone or hydrogen peroxide, or in a precipitation reaction of barium or lead nitrite with ammonium sulfate, or silver nitrite with ammonium chloride, or ammonium perchlorate with potassium nitrite. The precipitate is filtered off and the solution concentrated. It forms colorless crystals which are soluble in water and decompose on heating or in the presence of acid, with the formation of nitrogen.[3]
- NH4NO2 → N2 + 2 H2O
Properties
Ammonium nitrite may explode at a temperature of 60-70 °C.[2] It decomposes more quickly when a concentrated solution than when it is a dry crystal.
References
- ^ "Ammonium nitrite - Identification, toxicity, use, water pollution potential, ecological toxicity and regulatory information". PAN Pesticides Database - Chemicals. Retrieved 2006-07-26.
- ^ a b Thomas Scott; Mary Eagleson (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 66. ISBN 3110114518.
- ^ "VIAS Encyclopedia: Ammonium Nitrite".