Plasmic Physics (talk | contribs) No edit summary |
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: StdInChI StdInChIKey. |
||
| Line 10: | Line 10: | ||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 23201 |
| ChemSpiderID = 23201 |
||
| StdInChI = 1S/Cl4Si/c1-5(2,3)4 |
|||
| StdInChIKey = FDNAPBUWERUEDA-UHFFFAOYSA-N |
|||
| CASNo = 10026-04-7 |
| CASNo = 10026-04-7 |
||
| CASNo_Ref = {{cascite|correct|CAS}} |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
Revision as of 13:42, 29 November 2010
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Silicon (IV) chloride
| |||
| Other names
Silicon tetrachloride
Tetrachlorosilane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.037 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UN number | 1818 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SiCl4 | |||
| Molar mass | 169.90 g/mol | ||
| Appearance | Colourless liquid | ||
| Density | 1.483 g/cm3 | ||
| Melting point | −68.74 °C | ||
| Boiling point | 57.65 °C | ||
| decomposes | |||
| Solubility | soluble in benzene, toluene, chloroform, ether | ||
| Vapor pressure | 25.9 kPa at 20 °C | ||
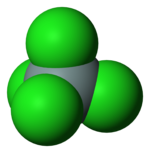
| Structure | |||
| Tetrahedral | |||
| 4 | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions
|
Silicon tetrafluoride Silicon tetrabromide Silicon tetraiodide | ||
Other cations
|
Carbon tetrachloride Germanium tetrachloride Tin(IV) chloride Titanium tetrachloride | ||
| Supplementary data page | |||
| Silicon tetrachloride (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Silicon tetrachloride is a non-polar chemical compound with the formula SiCl4. It was prepared by Jöns Jakob Berzelius in 1823.
Chemistry
This colorless volatile liquid compound is prepared by the treatment of silicon with chlorine:
- Si + 2 Cl2 → SiCl4
It reacts readily with water, in contrast with carbon tetrachloride. The differing rates of hydrolysis are attributed to the greater atomic radius of the silicon atom, whereas carbon has a smaller atomic radius so the chlorine atoms effectively shield the carbon from attack. In water, the following reaction occurs:
- SiCl4 + 2 H2O → SiO2 + 4 HCl
The reaction can be noticed on exposure of the liquid to air at room temperature: the vapour produces fumes as it reacts with moisture in the air.[1] With methanol and ethanol it reacts to give tetramethyl orthosilicate and tetraethyl orthosilicate:
- SiCl4 + 4 ROH → Si(OR)4 + 4 HCl
At higher temperatures homologues of silicon tetrachloride can be prepared by the reaction:
- Si + SiCl4 → Si2Cl6 + homologues
A series of compounds containing up to six silicon atoms in the chain can be separated from the mixture using fractional distillation.
Uses
Silicon tetrachloride is sometimes used as an intermediate in the manufacture of extremely pure silicon, since it has a boiling point convenient for purification by repeated fractional distillation; it can be reduced to silicon by hydrogen gas, or hydrolysed to SiO2 as a precursor for extremely pure synthetic fused silica. Very pure silicon derived from silicon tetrachloride is used in large amounts in the semiconductor industry, and also in the production of photovoltaic cells. Reports of silicon tetrachloride pollution in China have been associated with the increased demand for photovoltaic cells that has been stimulated by subsidy programs.[2]