Content deleted Content added
m category:Naphthoquinones |
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: InChI1->InChI StdInChI StdInChIKey. |
||
| Line 7: | Line 7: | ||
| ChemSpiderID = 9790 |
| ChemSpiderID = 9790 |
||
| PubChem = 10205 |
| PubChem = 10205 |
||
| |
| InChI = 1/C11H8O3/c1-6-5-9(13)10-7(11(6)14)3-2-4-8(10)12/h2-5,12H,1H3 |
||
| |
| InChIKey = VCMMXZQDRFWYSE-UHFFFAOYAB |
||
| StdInChI = 1S/C11H8O3/c1-6-5-9(13)10-7(11(6)14)3-2-4-8(10)12/h2-5,12H,1H3 |
|||
| StdInChIKey = VCMMXZQDRFWYSE-UHFFFAOYSA-N |
|||
| CASNo = 481-42-5 |
| CASNo = 481-42-5 |
||
| SMILES = O=C\2c1c(O)cccc1C(=O)/C(=C/2)C |
| SMILES = O=C\2c1c(O)cccc1C(=O)/C(=C/2)C |
||
Revision as of 13:31, 29 November 2010
| |
| Names | |
|---|---|
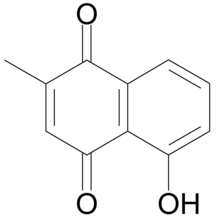
| IUPAC name
5-hydroxy-2-methyl-naphthalene-1,4-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.882 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H8O3 | |
| Molar mass | 188.17942 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Plumbagin or 5-hydroxy-2-methyl-1,4-naphthoquinone is an organic compound with the chemical formula C
11H
8O
3. It is regarded as a toxin.[1]
Plumbagin is a yellow dye,[1] formally derived from naphthoquinone, with a number of antimicrobial properties.[2][3] In animals, it has antimalarial,[4] anticarcinogenic,[5][6] cardiotonic,[7] antifertility action,[8] and anti-atherosclerosis effects.[9]
Plumbagin is named after the plant genus Plumbago, from which it was originally isolated.[10] It is also commonly found in the carnivorous plant genera Drosera and Nepenthes.[11]
References
- ^ a b Black Walnut. Drugs.com.
- ^ Didry, N., L. Dubrevil & M. Pinkas 1994. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Die Pharmazie 49(9): 681–683.
- ^ Paiva, S.R.d., M.R. Figueiredo, T. V. Aragão, M.A.C. Kaplan 2003. Template:PDFlink Mem Inst Oswaldo Cruz 98(7): 959–961.
- ^ Likhitwitayawuid, K., R. Kaewamatawong, N. Ruangrungsi & J. Krungkrai 1998. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Medica 64(3): 237–241.
- ^ Parimala, R. & P. Sachdanandam 1993. Effect of plumbagin on some glucose metabolizing enzymes studied in rats in experimental hepatoma. Molecular and Cellular Biochemistry 12(1): 59–63.
- ^ Hsu, Y.-L., C.-Y. Cho, P.-L. Kuo, Y.-T. Huang & C.-C. Lin 2006. Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) Induces Apoptosis and Cell Cycle Arrest in A549 Cells through p53 Accumulation via c-Jun NH2-Terminal Kinase-Mediated Phosphorylation at Serine 15 in Vitro and in Vivo. Journal of Pharmacology and Experimental Therapeutics 318(2): 484–494. doi:10.1124/jpet.105.098863
- ^ Itoigawa, M., K. Takeya & H. Furukawa 1991. Cardiotonic action of plumbagin on guinea-pig papillary muscle. Planta Medica 57(4): 317–319.
- ^ Bhargava, S.K. 1984. Effects of plumbagin on reproductive function of male dog. Indian Journal of Experimental Biology 22(3): 153–156.
- ^ Ding, Y., Z.-J. Chen, S. Liu, D. Che, M. Vetter, C.-H. Chang 2005. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. Journal of Pharmacy and Pharmacology 57(1): 111.
- ^ Van der Vijver, L.M. 1972. Distribution of plumbagin in the Plumbaginaceae. Phytochemistry 11: 3247–3248.
- ^ Wang, W., X. Luo, & H. Li 2010. Terahertz and infrared spectra of plumbagin, juglone, and menadione. Carnivorous Plant Newsletter 39(3): 82–88.