Content deleted Content added
Angusmclellan (talk | contribs) m update link for image moved to commons & renamed |
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChemSpiderID StdInChI StdInChIKey SMILES. |
||
| Line 6: | Line 6: | ||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| Abbreviations = |
| Abbreviations = |
||
| ChemSpiderID = 11483776 |
|||
| StdInChI = 1S/2Sb.3Se/q2*+3;3*-2 |
|||
| StdInChIKey = WWUNXXBCFXOXHC-UHFFFAOYSA-N |
|||
| CASNo = 1315-05-5 |
| CASNo = 1315-05-5 |
||
| EINECS = |
| EINECS = |
||
| PubChem = 6391662 |
| PubChem = 6391662 |
||
| SMILES = |
| SMILES = [SbH3+3].[SbH3+3].[Se-2].[Se-2].[Se-2] |
||
| InChI = |
| InChI = |
||
| RTECS = |
| RTECS = |
||
Revision as of 12:59, 29 November 2010
| |
| Names | |
|---|---|
| Other names
antimonselite
selenoxyantimony | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.870 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
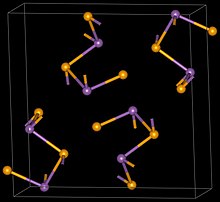
| Sb2Se3 | |
| Molar mass | 480.4 g/mol |
| Appearance | black crystals |
| Density | 5.81 g/cm3, solid |
| Melting point | 611 °C |
| Structure | |
| Orthorhombic, oP20, SpaceGroup = Pnma, No. 62 | |
| Related compounds | |
Other anions
|
antimony(III) oxide, antimony(III) sulfide, antimony(III) telluride |
Other cations
|
arsenic(III) selenide, bismuth(III) selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antimony triselenide is the chemical compound with the formula Sb2Se3. The material exists as the sulfosalt mineral antimonselite, which crystallizes in an orthorhombic space group.[1] In this compound, antimony is assigned the oxidation state 3+ and selenium 2-, but in fact the bonding in this compound is highly covalent as reflected by the black color and semiconducting properties of this and related materials.[2]
It may be formed by the reaction of antimony with selenium.
References
- ^ Jambor, J. L.; Grew, E. S."New Mineral Names" American Mineralogist, Volume 79, pages 387-391, 1994.
- ^ Caracas, R.; Gonze, X. "First-principles study of the electronic properties of A2B3 minerals,, with A=Bi,Sb and B=S,Se, Note: Hypothetical sulphosalt structure derived from density functional theory"" Physics and Chemistry of Minerals 2005, volume 32 p.295-300.