Content deleted Content added
m biflavonoid |
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: StdInChI StdInChIKey. |
||
| Line 12: | Line 12: | ||
| InChIKey = YUSWMAULDXZHPY-UHFFFAOYAB |
| InChIKey = YUSWMAULDXZHPY-UHFFFAOYAB |
||
| StdInChI = 1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-36H |
|||
| StdInChIKey = YUSWMAULDXZHPY-UHFFFAOYSA-N |
|||
| CASNo = 1617-53-4 |
| CASNo = 1617-53-4 |
||
Revision as of 12:00, 29 November 2010
| |
| Names | |
|---|---|
| IUPAC name
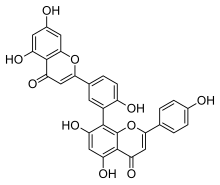
8-[5-(5,7-dihydroxy-4-oxo-chromen-2-yl)-2-hydroxy-phenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
| |
| Other names
Didemethyl-ginkgetin
3',8-Biapigenin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H18O10 | |
| Molar mass | 538.45 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amentoflavone is a constituent of a number of plants with medicinal properties, including Ginkgo biloba and Hypericum perforatum (St. John’s Wort).[1]
It is a biflavonoid (bis-apigenin coupled at 8 and 3' positions).
Amentoflavone can interact with many other medications by being a potent inhibitor of CYP3A4 and CYP2C9, which are proteins used for drug metabolism in the body.[2]
References
- ^ Pan X, Tan N, Zeng G, Zhang Y, Jia R (2005). "Amentoflavone and its derivatives as novel natural inhibitors of human Cathepsin B". Bioorg. Med. Chem. 13 (20): 5819–25. doi:10.1016/j.bmc.2005.05.071. PMID 16084098.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kimura Y, Ito H, Ohnishi R, Hatano T (2010). "Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity". Food Chem. Toxicol. 48 (1): 429–35. doi:10.1016/j.fct.2009.10.041. PMID 19883715.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)