| |
| Names | |
|---|---|
| IUPAC name
Europium(II) oxide
| |
| Other names
Europium monoxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.497 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| EuO | |
| Molar mass | 167.963 g/mol |
| Appearance | Violet crystals[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Europium(II) oxide (EuO) is a chemical compound which is one of the oxides of europium. In addition to europium(II) oxide, there is also europium(III) oxide and the mixed valence europium(II,III) oxide.
Preparation[edit]
Europium(II) oxide can be prepared by the reduction of europium(III) oxide with elemental europium at 800 °C and subsequent vacuum distillation at 1150 °C.[2]
- Eu2O3 + Eu → 3 EuO
It is also possible to synthesize from the reaction of europium oxychloride and lithium hydride.[3]
- 2 EuOCl + 2 LiH → 2 EuO + 2 LiCl + H2
In modern research, thin films can be manufactured by molecular beam epitaxy directly from europium atoms and oxygen molecules. These films have contamination of Eu3+ of less than 1%.[4][5]
Properties[edit]
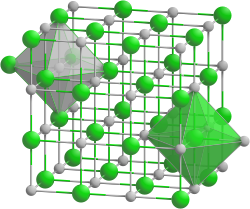
Europium(II) oxide is a violet compound as a bulk crystal and transparent blue in thin film form. It is unstable in humid atmosphere, slowly turning into the yellow europium(II) hydroxide hydrrate and then to white europium(III) hydroxide.[3] EuO crystallizes in a cubic sodium chloride structure with a lattice parameter a = 0.5144nm. The compound is often non-stoichiometric, containing up to 4% Eu3+ and small amounts of elemental europium.[6] However, since 2008 high purity crystalline EuO films can be created in ultra high vacuum conditions. These films have a crystallite size of about 4 nm.[citation needed]
Europium(II) oxide is ferromagnetic with a Curie Temperature of 69.3 K. With the addition of about 5-7% elemental europium, this increases to 79 K.[2] It also displays colossal magnetoresistance, with a dramatic increase in conductivity below the Curie temperature. One more way to increase the Curie temperature is doping with gadolinium, holmium, or lanthanum.[6]
Europium(II) oxide is a semiconductor with a band gap of 1.12 eV.[6]
Applications[edit]
Because of the properties of europium(II) oxide, thin layers of the oxide deposited on silicon are being studied for use as spin filters. Spin filter materials only allow electrons of a certain spin to pass, blocking electrons of the opposite spin.[7]
References[edit]
- ^ McGill, Ian (2000-06-15), "Rare Earth Elements", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, doi:10.1002/14356007.a22_607, ISBN 3527306730
- ^ a b Shafer, M. W. (1965). "Preparation and Crystal Chemistry of Divalent Europium Compounds". Journal of Applied Physics. 36 (3). AIP Publishing: 1145–1152. Bibcode:1965JAP....36.1145S. doi:10.1063/1.1714142. ISSN 0021-8979.
- ^ a b Baudler, Marianne (1978). Handbuch der präparativen anorganischen Chemie (in German). Stuttgart: Enke. p. 1092. ISBN 3-432-87813-3. OCLC 310719490.
- ^ Sutarto, R.; Altendorf, S. G.; Coloru, B.; Moretti Sala, M.; Haupricht, T.; et al. (2009-05-21). "Epitaxial and layer-by-layer growth of EuO thin films on yttria-stabilized cubic zirconia (001) using MBE distillation". Physical Review B. 79 (20): 205318. arXiv:0902.0330. Bibcode:2009PhRvB..79t5318S. doi:10.1103/physrevb.79.205318. ISSN 1098-0121. S2CID 97016104.
- ^ Altendorf, S. G.; Efimenko, A.; Oliana, V.; Kierspel, H.; Rata, A. D.; Tjeng, L. H. (2011-10-28). "Oxygen off-stoichiometry and phase separation in EuO thin films". Physical Review B. 84 (15). American Physical Society (APS): 155442. Bibcode:2011PhRvB..84o5442A. doi:10.1103/physrevb.84.155442. ISSN 1098-0121.
- ^ a b c Santos, Tiffany S. (2008-11-10). Europium oxide as a perfect electron spin filter. DSpace@MIT Home (Thesis). Massachusetts Institute of Technology. hdl:1721.1/39538. Retrieved 2022-01-23.
- ^ Caspers, C.; Müller, M.; Gray, A. X.; Kaiser, A. M.; Gloskovskii, A.; Fadley, C. S.; Drube, W.; Schneider, C. M. (2011-10-12). "Electronic structure of EuO spin filter tunnel contacts directly on silicon" (PDF). Physica Status Solidi RRL. 5 (12). Wiley: 441–443. Bibcode:2011PSSRR...5..441C. doi:10.1002/pssr.201105403. ISSN 1862-6254. S2CID 22764388.