This page provides supplementary chemical data on butanone.
Material Safety Data Sheet[edit]
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties[edit]
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.3807 at 15.9 °C |
| Abbe number | 32 |
| Dielectric constant, εr | 18.5 ε0 at 20 °C |
| Bond strength | 2.67 pa |
| Bond length | r. |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension | 23.9 dyn/cm at 25 °C |
| Viscosity[1] | 0.5429 mPa·s at 0 °C 0.4284 mPa·s at 20 °C 0.3490 mPa·s at 40 °C 0.2482 mPa·s at 80 °C |
Thermodynamic properties[edit]
| Phase behavior | |
|---|---|
| Triple point | 186.5 K (–86.5 °C), ? Pa |
| Critical point | 533 K (260 °C), 4.002 MPa |
| Std enthalpy change of fusion, ΔfusH |
8.44 kJ/mol |
| Std entropy change of fusion, ΔfusS |
44.98 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
32.2 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
91.6 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–273.3 kJ/mol |
| Standard molar entropy, S |
239.0 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–2444.2 kJ/mol |
| Heat capacity, cp | 158.4 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–238.5 kJ/mol |
| Standard molar entropy, S |
310 J/(mol K) |
| Heat capacity, cp | 101.68 J/(mol K) at 25 °C |
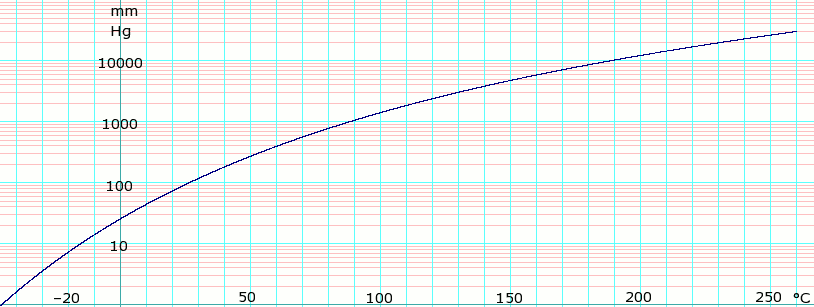
Vapor pressure of liquid[edit]
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | –48.3 | –17.7 | 6.0 | 25.0 | 60.0 | 79.6 | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
Spectral data[edit]
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
References[edit]
- ^ Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- ^ "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 8 May 2007.
This box:
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
