Biochemistry&Love (talk | contribs) Copy edit Tags: Visual edit Mobile edit Mobile web edit |
JJMC89 bot III (talk | contribs) m Moving Category:4-Morpholinyl compunds to Category:4-Morpholinyl compounds per Wikipedia:Categories for discussion/Speedy |
||
| (19 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
|||
{{Infobox drug |
|||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 447554700 |
| verifiedrevid = 447554700 |
||
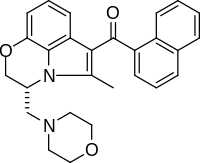
| IUPAC_name = (11''R'')-2- |
| IUPAC_name = (11''R'')-2-Methyl-11-[(morpholin-4-yl)methyl]-3-(naphthalene-1-carbonyl)-9-oxa-1-azatricyclo[6.3.1.0<sup>4,12</sup>]dodeca-2,4(12),5,7-tetraene |
||
| image = WIN 55,212-2-2D-skeletal.svg |
| image = WIN 55,212-2-2D-skeletal.svg |
||
| width = 200px |
| width = 200px |
||
| Line 9: | Line 10: | ||
<!--Clinical data--> |
<!--Clinical data--> |
||
| tradename = |
| tradename = |
||
| legal_CA = Schedule II <ref>{{cite web | title = Controlled Drugs and Substance Act - Schedule II | url = https://laws-lois.justice.gc.ca/eng/acts/c-38.8/page-14.html#docCont | work = Justice Laws Website | date = 18 March 2021 | publisher = Government of Canada }}</ref> |
|||
| ⚫ | |||
| legal_UK = Class B |
| legal_UK = Class B |
||
| ⚫ | |||
| legal_status = |
| legal_status = |
||
| routes_of_administration = |
| routes_of_administration = |
||
| Line 35: | Line 37: | ||
<!--Chemical data--> |
<!--Chemical data--> |
||
| C=27 | H=26 | N=2 | O=3 |
| C=27 | H=26 | N=2 | O=3 |
||
| molecular_weight = 426.52 g/mol |
|||
| smiles = CC1=C(C2=C3N1[C@@H](COC3=CC=C2)CN4CCOCC4)C(=O)C5=CC=CC6=CC=CC=C65 |
| smiles = CC1=C(C2=C3N1[C@@H](COC3=CC=C2)CN4CCOCC4)C(=O)C5=CC=CC6=CC=CC=C65 |
||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
||
| Line 43: | Line 44: | ||
}} |
}} |
||
[[File:Pancreatic stellate cell cropped.png|thumb|right|[[Pancreatic stellate cell]]s. The cells in the lower frame are under the action of WIN 55,212-2. They are thought to assume a more "[[G0 phase|quiescent]]" phenotype. From Michalski et al., 2008.<ref name="PSCWIN">{{ |
[[File:Pancreatic stellate cell cropped.png|thumb|right|[[Pancreatic stellate cell]]s. The cells in the lower frame are under the action of WIN 55,212-2. They are thought to assume a more "[[G0 phase|quiescent]]" phenotype. From Michalski et al., 2008.<ref name="PSCWIN">{{cite journal | vauthors = Michalski CW, Maier M, Erkan M, Sauliunaite D, Bergmann F, Pacher P, Batkai S, Giese NA, Giese T, Friess H, Kleeff J | display-authors = 6 | title = Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells | journal = PLOS ONE | volume = 3 | issue = 2 | pages = e1701 | date = February 2008 | pmid = 18301776 | pmc = 2253501 | doi = 10.1371/journal.pone.0001701 | veditors = Gluud C | doi-access = free | bibcode = 2008PLoSO...3.1701M }}</ref>]] |
||
| ⚫ | '''WIN 55,212-2''' is a chemical described as an [[aminoalkylindole]] derivative, which produces effects similar to those of [[cannabinoid]]s such as [[tetrahydrocannabinol]] (THC) but has an entirely different [[chemical structure]].<ref>{{cite journal | vauthors = Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR | title = Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 263 | issue = 3 | pages = 1118–1126 | date = December 1992 | pmid = 1335057 }}</ref><ref>{{cite journal | vauthors = Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T | title = The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: an in vivo and in vitro study | journal = Cerebral Cortex | volume = 11 | issue = 8 | pages = 728–733 | date = August 2001 | pmid = 11459762 | doi = 10.1093/cercor/11.8.728 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Zhang Q, Ma P, Iszard M, Cole RB, Wang W, Wang G | title = In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist | journal = Drug Metabolism and Disposition | volume = 30 | issue = 10 | pages = 1077–1086 | date = October 2002 | pmid = 12228183 | doi = 10.1124/dmd.30.10.1077 | s2cid = 10848076 }} |
||
</ref>]] |
|||
| ⚫ | '''WIN 55,212-2''' is a chemical described as an [[aminoalkylindole]] derivative, which produces effects similar to those of [[cannabinoid]]s such as [[tetrahydrocannabinol]] (THC) but has an entirely different [[chemical structure]].<ref>{{cite journal | |
||
</ref> |
</ref> |
||
WIN 55,212-2 is a potent cannabinoid [[receptor agonist]]<ref>{{cite journal | vauthors = Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL | display-authors = 6 | title = Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors | journal = Molecular Pharmacology | volume = 48 | issue = 3 | pages = 443–450 | date = September 1995 | pmid = 7565624 }}</ref> that has been found to be a potent analgesic<ref>{{cite journal | vauthors = Meng ID, Manning BH, Martin WJ, Fields HL | title = An analgesia circuit activated by cannabinoids | journal = Nature | volume = 395 | issue = 6700 | pages = 381–383 | date = September 1998 | pmid = 9759727 | doi = 10.1038/26481 | s2cid = 1619608 | bibcode = 1998Natur.395..381M }}</ref> in a rat model of neuropathic pain.<ref>{{cite journal | vauthors = Herzberg U, Eliav E, Bennett GJ, Kopin IJ | title = The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain | journal = Neuroscience Letters | volume = 221 | issue = 2–3 | pages = 157–160 | date = January 1997 | pmid = 9121688 | doi = 10.1016/S0304-3940(96)13308-5 | s2cid = 33643599 }}</ref> It activates [[p42 MAP kinase|p42]] and [[p44 MAP kinase|p44]] [[MAP kinase]] via receptor-mediated signaling.<ref>{{cite journal | vauthors = Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P | display-authors = 6 | title = Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1 | journal = The Biochemical Journal | volume = 312 ( Pt 2) | issue = Pt 2 | pages = 637–641 | date = December 1995 | pmid = 8526880 | pmc = 1136308 | doi = 10.1042/bj3120637 }}</ref> |
|||
WIN 55,212-2 is a potent cannabinoid [[receptor agonist]]<ref>{{Cite journal |
|||
| last1 = Felder | first1 = C. C. |
|||
| last2 = Joyce | first2 = K. E. |
|||
| last3 = Briley | first3 = E. M. |
|||
| last4 = Mansouri | first4 = J. |
|||
| last5 = MacKie | first5 = K. |
|||
| last6 = Blond | first6 = O. |
|||
| last7 = Lai | first7 = Y. |
|||
| last8 = Ma | first8 = A. L. |
|||
| last9 = Mitchell | first9 = R. L. |
|||
| title = Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors |
|||
| journal = Molecular Pharmacology |
|||
| volume = 48 |
|||
| issue = 3 |
|||
| pages = 443–450 |
|||
| year = 1995 |
|||
| pmid = 7565624 |
|||
}}</ref> that has been found to be a potent analgesic<ref>{{Cite journal | last1 = Meng | first1 = I. D. | last2 = Manning | first2 = B. H. | last3 = Martin | first3 = W. J. | last4 = Fields | first4 = H. L. | title = An analgesia circuit activated by cannabinoids | journal = Nature | volume = 395 | issue = 6700 | pages = 381–383 | year = 1998 | doi = 10.1038/26481 | pmid = 9759727| pmc = }}</ref> in a rat model of neuropathic pain.<ref>{{Cite journal | last1 = Herzberg | first1 = U. | last2 = Eliav | first2 = E. | last3 = Bennett | first3 = G. J. | last4 = Kopin | first4 = I. J. | title = The analgesic effects of R(+)-WIN 55,212–2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain | doi = 10.1016/S0304-3940(96)13308-5 | journal = Neuroscience Letters | volume = 221 | issue = 2–3 | pages = 157–160 | year = 1997 | pmid = 9121688| pmc = }}</ref> It activates [[p42 MAP kinase|p42]] and [[p44 MAP kinase|p44]] [[MAP kinase]] via receptor-mediated signaling.<ref>{{Cite journal |
|||
| last1 = Bouaboula | first1 = M. |
|||
| last2 = Poinot-Chazel | first2 = C. |
|||
| last3 = Bourrié | first3 = B. |
|||
| last4 = Canat | first4 = X. |
|||
| last5 = Calandra | first5 = B. |
|||
| last6 = Rinaldi-Carmona | first6 = M. |
|||
| last7 = Le Fur | first7 = G. |
|||
| last8 = Casellas | first8 = P. |
|||
| title = Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1 |
|||
| journal = The Biochemical Journal |
|||
| volume = 312 |
|||
| issue = Pt 2 |
|||
| pages = 637–641 |
|||
| year = 1995 |
|||
| pmid = 8526880 |
|||
| pmc = 1136308 | doi=10.1042/bj3120637 |
|||
}}</ref> |
|||
At 5 |
At 5 μM WIN 55,212-2 inhibits [[Adenosine Triphosphate|ATP]] production in [[sperm]] in a [[CB1 receptor|CB<sub>1</sub> receptor]]-dependent fashion.<ref>{{cite journal | vauthors = Morgan DJ, Muller CH, Murataeva NA, Davis BJ, Mackie K | title = Δ9-Tetrahydrocannabinol (Δ9-THC) attenuates mouse sperm motility and male fecundity | journal = British Journal of Pharmacology | volume = 165 | issue = 8 | pages = 2575–2583 | date = April 2012 | pmid = 21615727 | pmc = 3423255 | doi = 10.1111/j.1476-5381.2011.01506.x }} |
||
</ref> |
</ref> |
||
WIN 55,212-2, along with [[HU-210]] and [[JWH-133]], may prevent the inflammation caused by [[amyloid beta]] proteins involved in [[Alzheimer's disease]], in addition to preventing cognitive impairment and loss of neuronal [[genetic markers|markers]]. This anti-inflammatory action is induced through agonist action at [[cannabinoid receptor]]s, which prevents [[microglia]]l activation that elicits the inflammation. |
WIN 55,212-2, along with [[HU-210]] and [[JWH-133]], may prevent the inflammation caused by [[amyloid beta]] proteins involved in [[Alzheimer's disease]], in addition to preventing cognitive impairment and loss of neuronal [[genetic markers|markers]]. This anti-inflammatory action is induced through agonist action at [[cannabinoid receptor]]s, which prevents [[microglia]]l activation that elicits the inflammation. |
||
WIN 55,212-2 is a full agonist at the [[CB1 receptor|CB<sub>1</sub> cannabinoid receptor]] ([[Dissociation constant|''K''<sub>i</sub>]] = 1.9 nM) and has much higher affinity than [[tetrahydrocannabinol|THC]] (''K''<sub>i</sub> = 41 nM) for this receptor.<ref>{{ |
WIN 55,212-2 is a full agonist at the [[CB1 receptor|CB<sub>1</sub> cannabinoid receptor]] ([[Dissociation constant|''K''<sub>i</sub>]] = 1.9 nM) and has much higher affinity than [[tetrahydrocannabinol|THC]] (''K''<sub>i</sub> = 41 nM) for this receptor.<ref>{{cite journal | vauthors = Kuster JE, Stevenson JI, Ward SJ, D'Ambra TE, Haycock DA | title = Aminoalkylindole binding in rat cerebellum: selective displacement by natural and synthetic cannabinoids | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 264 | issue = 3 | pages = 1352–1363 | date = March 1993 | pmid = 8450470 }} |
||
</ref> WIN 55,212-2 is also an agonist of the [[ |
</ref> WIN 55,212-2 is also an agonist of the [[Peroxisome proliferator-activated receptor alpha|PPARα]] and [[Peroxisome proliferator-activated receptor gamma|PPARγ]] nuclear receptors.<ref name="pmid27077495">{{cite journal | vauthors = O'Sullivan SE | title = An update on PPAR activation by cannabinoids | journal = British Journal of Pharmacology | volume = 173 | issue = 12 | pages = 1899–1910 | date = June 2016 | pmid = 27077495 | pmc = 4882496 | doi = 10.1111/bph.13497 }}</ref> |
||
WIN 55,212-2 reduces voluntary wheel running in laboratory mice, but with effects that depend on both genetic background and sex.<ref>{{cite journal |
WIN 55,212-2 reduces voluntary wheel running in laboratory mice, but with effects that depend on both genetic background and sex.<ref>{{cite journal | vauthors = Keeney BK, Meek TH, Middleton KM, Holness LF, Garland T | title = Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior | journal = Pharmacology, Biochemistry, and Behavior | volume = 101 | issue = 4 | pages = 528–537 | date = June 2012 | pmid = 22405775 | doi = 10.1016/j.pbb.2012.02.017 | s2cid = 25174208 }}</ref> |
||
WIN 55,212-2 is illegal in the UK.<ref name="legislation.gov.uk">{{cite web|url=http://www.legislation.gov.uk/uksi/2013/239/article/4/made|title=The Misuse of Drugs Act 1971 (Amendment) Order 2013}}</ref> |
In the United States, all CB<sub>1</sub> receptor agonists of the 3-(1-naphthoyl)indole class such as WIN 55,212-2 are [[Schedule I Controlled Substance]]s.<ref>{{UnitedStatesCode2|21|812|Schedules of controlled substances}}</ref> WIN 55,212-2 is illegal in the UK.<ref name="legislation.gov.uk">{{cite web|url=http://www.legislation.gov.uk/uksi/2013/239/article/4/made|title=The Misuse of Drugs Act 1971 (Amendment) Order 2013 | work = legislation.gov.uk }}</ref> |
||
WIN 55,212-2 is also a [[Cannabinoid receptor type 2|CB2 receptor]] agonist and thereby, like other cannabinoid CB2 agonists, found to significantly improve [[Heart|cardiac]] recovery after [[Ischemia|ischaemia]]/[[Reperfusion injury|reperfusion]] (I/R) in the hearts of [[Diabetes|diabetic]] [[Obesity|fatty]] rats, by restoring [[coronary perfusion pressure]] and [[heart rate]] to pre-[[Coronary artery disease|ischaemic]] levels, by the restoration of the [[inducible nitric oxide synthase]] (iNOS)/[[endothelial nitric oxide synthase]] (eNOS) cardiac [[Equilibrium constant|equilibrium]].<ref>{{cite journal | vauthors = González C, Herradón E, Abalo R, Vera G, Pérez-Nievas BG, Leza JC, Martín MI, López-Miranda V | display-authors = 6 | title = Cannabinoid/agonist WIN 55,212-2 reduces cardiac ischaemia–reperfusion injury in Zucker diabetic fatty rats: role of CB2 receptors and iNOS/eNOS | journal = Diabetes/Metabolism Research and Reviews | volume = 27 | issue = 4 | pages = 331–340 | date = May 2011 | pmid = 21309057 | doi = 10.1002/dmrr.1176 | s2cid = 32450365 }}</ref><ref>{{cite journal | vauthors = Shmist YA, Goncharov I, Eichler M, Shneyvays V, Isaac A, Vogel Z, Shainberg A | title = Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production | journal = Molecular and Cellular Biochemistry | volume = 283 | issue = 1–2 | pages = 75–83 | date = February 2006 | pmid = 16444588 | doi = 10.1007/s11010-006-2346-y | s2cid = 24074568 }}</ref> |
|||
| ⚫ | |||
| ⚫ | |||
* [[WIN 48,098]] (Pravadoline) |
* [[WIN 48,098]] (Pravadoline) |
||
* [[WIN 54,461]] (6-Bromopravadoline) |
* [[WIN 54,461]] (6-Bromopravadoline) |
||
| Line 105: | Line 73: | ||
{{reflist}} |
{{reflist}} |
||
== Further reading == |
|||
| ⚫ | |||
{{refbegin}} |
|||
| ⚫ | |||
* The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 ( |
* {{cite journal | vauthors = Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM | title = The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 103 | issue = 30 | pages = 11393–11398 | date = July 2006 | pmid = 16849427 | pmc = 1544096 | doi = 10.1073/pnas.0603861103 | doi-access = free | bibcode = 2006PNAS..10311393P }} |
||
* {{cite journal | vauthors = Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML | title = Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation | journal = The Journal of Neuroscience | volume = 25 | issue = 8 | pages = 1904–1913 | date = February 2005 | pmid = 15728830 | pmc = 6726060 | doi = 10.1523/JNEUROSCI.4540-04.2005 }} |
|||
* [http://www.jneurosci.org/cgi/content/full/25/8/1904?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=1&andorexacttitle=and&andorexacttitleabs=and&fulltext=cannabinoid+alzheimer%27s&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT JNeurosci.org] Prevention of Alzheimer's Disease Pathology by Cannabinoids: Neuroprotection Mediated by Blockade of Microglial Activation |
|||
{{refend}} |
|||
* [https://www.newscientist.com/article.ns?id=dn10330 New Scientist]: Hope for cannabis-based drug for Alzheimer's |
|||
| ⚫ | |||
| ⚫ | |||
{{Cannabinoids}} |
{{Cannabinoids}} |
||
| Line 117: | Line 88: | ||
[[Category:Aminoalkylindoles]] |
[[Category:Aminoalkylindoles]] |
||
[[Category: |
[[Category:4-Morpholinyl compounds]] |
||
[[Category:Morpholines]] |
|||
[[Category:Naphthoylindoles]] |
[[Category:Naphthoylindoles]] |
||
[[Category:WIN compounds]] |
[[Category:WIN compounds]] |
||
Latest revision as of 13:20, 10 July 2023
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H26N2O3 |
| Molar mass | 426.516 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.[3][4][5]
WIN 55,212-2 is a potent cannabinoid receptor agonist[6] that has been found to be a potent analgesic[7] in a rat model of neuropathic pain.[8] It activates p42 and p44 MAP kinase via receptor-mediated signaling.[9]
At 5 μM WIN 55,212-2 inhibits ATP production in sperm in a CB1 receptor-dependent fashion.[10]
WIN 55,212-2, along with HU-210 and JWH-133, may prevent the inflammation caused by amyloid beta proteins involved in Alzheimer's disease, in addition to preventing cognitive impairment and loss of neuronal markers. This anti-inflammatory action is induced through agonist action at cannabinoid receptors, which prevents microglial activation that elicits the inflammation.
WIN 55,212-2 is a full agonist at the CB1 cannabinoid receptor (Ki = 1.9 nM) and has much higher affinity than THC (Ki = 41 nM) for this receptor.[11] WIN 55,212-2 is also an agonist of the PPARα and PPARγ nuclear receptors.[12]
WIN 55,212-2 reduces voluntary wheel running in laboratory mice, but with effects that depend on both genetic background and sex.[13]
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as WIN 55,212-2 are Schedule I Controlled Substances.[14] WIN 55,212-2 is illegal in the UK.[15]
WIN 55,212-2 is also a CB2 receptor agonist and thereby, like other cannabinoid CB2 agonists, found to significantly improve cardiac recovery after ischaemia/reperfusion (I/R) in the hearts of diabetic fatty rats, by restoring coronary perfusion pressure and heart rate to pre-ischaemic levels, by the restoration of the inducible nitric oxide synthase (iNOS)/endothelial nitric oxide synthase (eNOS) cardiac equilibrium.[16][17]
See also[edit]
- WIN 48,098 (Pravadoline)
- WIN 54,461 (6-Bromopravadoline)
- WIN 55,225 (JWH-200)
- WIN 56,098
References[edit]
- ^ "Controlled Drugs and Substance Act - Schedule II". Justice Laws Website. Government of Canada. 18 March 2021.
- ^ Michalski CW, Maier M, Erkan M, Sauliunaite D, Bergmann F, Pacher P, et al. (February 2008). Gluud C (ed.). "Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells". PLOS ONE. 3 (2): e1701. Bibcode:2008PLoSO...3.1701M. doi:10.1371/journal.pone.0001701. PMC 2253501. PMID 18301776.
- ^ Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR (December 1992). "Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol". The Journal of Pharmacology and Experimental Therapeutics. 263 (3): 1118–1126. PMID 1335057.
- ^ Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T (August 2001). "The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: an in vivo and in vitro study". Cerebral Cortex. 11 (8): 728–733. doi:10.1093/cercor/11.8.728. PMID 11459762.
- ^ Zhang Q, Ma P, Iszard M, Cole RB, Wang W, Wang G (October 2002). "In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist". Drug Metabolism and Disposition. 30 (10): 1077–1086. doi:10.1124/dmd.30.10.1077. PMID 12228183. S2CID 10848076.
- ^ Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. (September 1995). "Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors". Molecular Pharmacology. 48 (3): 443–450. PMID 7565624.
- ^ Meng ID, Manning BH, Martin WJ, Fields HL (September 1998). "An analgesia circuit activated by cannabinoids". Nature. 395 (6700): 381–383. Bibcode:1998Natur.395..381M. doi:10.1038/26481. PMID 9759727. S2CID 1619608.
- ^ Herzberg U, Eliav E, Bennett GJ, Kopin IJ (January 1997). "The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain". Neuroscience Letters. 221 (2–3): 157–160. doi:10.1016/S0304-3940(96)13308-5. PMID 9121688. S2CID 33643599.
- ^ Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, et al. (December 1995). "Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1". The Biochemical Journal. 312 ( Pt 2) (Pt 2): 637–641. doi:10.1042/bj3120637. PMC 1136308. PMID 8526880.
- ^ Morgan DJ, Muller CH, Murataeva NA, Davis BJ, Mackie K (April 2012). "Δ9-Tetrahydrocannabinol (Δ9-THC) attenuates mouse sperm motility and male fecundity". British Journal of Pharmacology. 165 (8): 2575–2583. doi:10.1111/j.1476-5381.2011.01506.x. PMC 3423255. PMID 21615727.
- ^ Kuster JE, Stevenson JI, Ward SJ, D'Ambra TE, Haycock DA (March 1993). "Aminoalkylindole binding in rat cerebellum: selective displacement by natural and synthetic cannabinoids". The Journal of Pharmacology and Experimental Therapeutics. 264 (3): 1352–1363. PMID 8450470.
- ^ O'Sullivan SE (June 2016). "An update on PPAR activation by cannabinoids". British Journal of Pharmacology. 173 (12): 1899–1910. doi:10.1111/bph.13497. PMC 4882496. PMID 27077495.
- ^ Keeney BK, Meek TH, Middleton KM, Holness LF, Garland T (June 2012). "Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior". Pharmacology, Biochemistry, and Behavior. 101 (4): 528–537. doi:10.1016/j.pbb.2012.02.017. PMID 22405775. S2CID 25174208.
- ^ : Schedules of controlled substances
- ^ "The Misuse of Drugs Act 1971 (Amendment) Order 2013". legislation.gov.uk.
- ^ González C, Herradón E, Abalo R, Vera G, Pérez-Nievas BG, Leza JC, et al. (May 2011). "Cannabinoid/agonist WIN 55,212-2 reduces cardiac ischaemia–reperfusion injury in Zucker diabetic fatty rats: role of CB2 receptors and iNOS/eNOS". Diabetes/Metabolism Research and Reviews. 27 (4): 331–340. doi:10.1002/dmrr.1176. PMID 21309057. S2CID 32450365.
- ^ Shmist YA, Goncharov I, Eichler M, Shneyvays V, Isaac A, Vogel Z, Shainberg A (February 2006). "Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production". Molecular and Cellular Biochemistry. 283 (1–2): 75–83. doi:10.1007/s11010-006-2346-y. PMID 16444588. S2CID 24074568.
Further reading[edit]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM (July 2006). "The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin". Proceedings of the National Academy of Sciences of the United States of America. 103 (30): 11393–11398. Bibcode:2006PNAS..10311393P. doi:10.1073/pnas.0603861103. PMC 1544096. PMID 16849427.
- Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML (February 2005). "Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation". The Journal of Neuroscience. 25 (8): 1904–1913. doi:10.1523/JNEUROSCI.4540-04.2005. PMC 6726060. PMID 15728830.
External links[edit]
- "Win 55,212-2 Data Sheet". Enzo Life Sciences.