Content deleted Content added
Updating {{drugbox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia talk:Wi |
populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot |
||
| Line 1: | Line 1: | ||
{{Drugbox |
{{Drugbox |
||
| verifiedrevid = 399138585 |
| verifiedrevid = 399138585 |
||
| IUPAC_name |
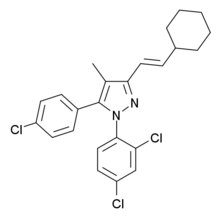
| IUPAC_name = 5-(4-chlorophenyl)- 3-[(E)-2-cyclohexylethenyl]- 1-(2,4-dichlorophenyl)- 4-methyl- 1H-pyrazole |
||
| image |
| image = VCHSR.png |
||
| ⚫ | |||
<!--Clinical data--> |
|||
| ⚫ | |||
| |
| tradename = |
||
| |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| |
| pregnancy_US = <!-- A / B / C / D / X --> |
||
| pregnancy_category = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| protein_bound = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Pharmacokinetic data--> |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| ⚫ | |||
| pregnancy_US = <!-- A / B / C / D / X --> |
|||
| |
| protein_bound = |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Identifiers--> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ATC_suffix = |
|||
| PubChem = |
|||
| DrugBank = |
|||
<!--Chemical data--> |
|||
| ⚫ | |||
| C=24 | H=23 | Cl=3 | N=2 |
|||
| ⚫ | |||
| ⚫ | |||
}} |
}} |
||
Revision as of 20:24, 2 September 2011
 | |
| Identifiers | |
|---|---|
| |
| Chemical and physical data | |
| Formula | C24H23Cl3N2 |
| Molar mass | 445.811 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
VCHSR is a drug used in scientific research which acts as a selective antagonist of the cannabinoid receptor CB1. It is derived from the widely used CB1 antagonist rimonabant, and has similar potency and selectivity for the CB1 receptor, but has been modified to remove the hydrogen bonding capability in the C-3 substituent region, which removes the inverse agonist effect that rimonabant produces at high doses, so that VCHSR instead acts as a neutral antagonist, blocking the receptor but producing no physiological effect of its own.[1][2]
References
- ^ Hurst, DP; Lynch, DL; Barnett-Norris, J; Hyatt, SM; Seltzman, HH; Zhong, M; Song, ZH; Nie, J; Lewis, D (2002). "N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor". Molecular pharmacology. 62 (6): 1274–87. PMID 12435794.
- ^ Hurst, D; Umejiego, U; Lynch, D; Seltzman, H; Hyatt, S; Roche, M; McAllister, S; Fleischer, D; Kapur, A (2006). "Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction". Journal of medicinal chemistry. 49 (20): 5969–87. doi:10.1021/jm060446b. PMID 17004712.