89.240.140.133 (talk) |
remove content added by sock of blocked user Nuklear |
||
| Line 68: | Line 68: | ||
'''Tropinone''' is an [[alkaloid]], famously synthesised in 1917 by [[Robert Robinson (organic chemist)|Robert Robinson]] as a [[organic chemistry|synthetic]] precursor to [[atropine]], a scarce commodity during [[World War I]].<ref>{{cite doi|10.1039/CT9171100762}}</ref><ref>{{Cite pmid|10649349}}</ref> Tropinone and the alkaloids [[cocaine]] and atropine all share the same [[tropane]] core structure. |
'''Tropinone''' is an [[alkaloid]], famously synthesised in 1917 by [[Robert Robinson (organic chemist)|Robert Robinson]] as a [[organic chemistry|synthetic]] precursor to [[atropine]], a scarce commodity during [[World War I]].<ref>{{cite doi|10.1039/CT9171100762}}</ref><ref>{{Cite pmid|10649349}}</ref> Tropinone and the alkaloids [[cocaine]] and atropine all share the same [[tropane]] core structure. |
||
== |
==Synthesis== |
||
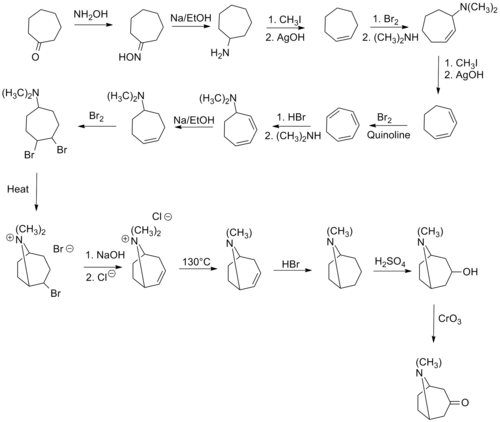
The first synthesis of tropinone was by [[Richard Willstätter]] in 1901. It started from the seemingly related [[cycloheptanone]], but required many steps to introduce the nitrogen bridge; the overall [[yield (chemistry)|yield]] for the synthesis path is only 0.75%.<ref name=smit>{{Cite doi|10.1039/9781847551573}}</ref> Willstätter had previously synthesized cocaine from tropinone, in what was the first synthesis and elucidation of the structure of cocaine.<ref>{{Cite doi|10.1039/b001713m}}</ref> |
The first synthesis of tropinone was by [[Richard Willstätter]] in 1901. It started from the seemingly related [[cycloheptanone]], but required many steps to introduce the nitrogen bridge; the overall [[yield (chemistry)|yield]] for the synthesis path is only 0.75%.<ref name=smit>{{Cite doi|10.1039/9781847551573}}</ref> Willstätter had previously synthesized cocaine from tropinone, in what was the first synthesis and elucidation of the structure of cocaine.<ref>{{Cite doi|10.1039/b001713m}}</ref> |
||
[[File: |
[[File:Willstatter tropinone synthesis.png|500px]]<ref>{{cite book |first1=Mukesh |last1=Doble |first2=Anil Kumar |last2=Kruthiventi |title=Green Chemistry and Engineering |year=2007 |publisher=Elsevier |location=Oxford |isbn=978-0-12-372532-5 |pages=34}}</ref> |
||
==Robinson Synthesis== |
|||
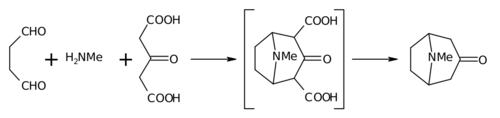
The 1917 synthesis by Robinson is considered a classic in [[total synthesis]]<ref>{{Cite doi|10.1098/rsnr.1993.0034}}</ref> due to its simplicity and biomimetic approach. Tropinone is a [[bicyclic molecule]], but the [[reactant]]s used in its preparation are fairly simple: [[succinaldehyde]], [[methylamine]] and [[acetonedicarboxylic acid]] (or even [[acetone]]). The synthesis is a good example of a [[biomimetic]] reaction or '''biogenetic-type synthesis''' because [[biosynthesis]] makes use of the same building blocks. It also demonstrates a [[tandem reaction]] in a [[one-pot synthesis]]. Furthermore the yield of the synthesis was 17% and with subsequent improvements exceeded 90%.<ref name=smit /> |
The 1917 synthesis by Robinson is considered a classic in [[total synthesis]]<ref>{{Cite doi|10.1098/rsnr.1993.0034}}</ref> due to its simplicity and biomimetic approach. Tropinone is a [[bicyclic molecule]], but the [[reactant]]s used in its preparation are fairly simple: [[succinaldehyde]], [[methylamine]] and [[acetonedicarboxylic acid]] (or even [[acetone]]). The synthesis is a good example of a [[biomimetic]] reaction or '''biogenetic-type synthesis''' because [[biosynthesis]] makes use of the same building blocks. It also demonstrates a [[tandem reaction]] in a [[one-pot synthesis]]. Furthermore the yield of the synthesis was 17% and with subsequent improvements exceeded 90%.<ref name=smit /> |
||
:[[Image:Robinson tropinone synthesis.png|500px]] |
:[[Image:Robinson tropinone synthesis.png|500px]] |
||
This reaction is described as an intramolecular "[[ |
This reaction is described as an intramolecular "double [[Mannich reaction]]" for obvious reasons. It is not unique in this regard, as others have also attempted it in piperidine synthesis.<ref>{{Cite pmid|10669562}}</ref><ref>{{Cite pmid|11425577}}</ref> |
||
In place of acetone, acetonedicarboxylic acid is known as the "[[synthetic equivalent]]" the 1,3-dicarboxylic acid groups are so-called "[[activating group]]s" to facilitate the ring forming reactions. The calcium salt is there as a "[[Buffer solution|buffer]]" as it is claimed that higher yields are possible if the reaction is conducted at "[[physiological]] [[pH]]". |
In place of acetone, acetonedicarboxylic acid is known as the "[[synthetic equivalent]]" the 1,3-dicarboxylic acid groups are so-called "[[activating group]]s" to facilitate the ring forming reactions. The calcium salt is there as a "[[Buffer solution|buffer]]" as it is claimed that higher yields are possible if the reaction is conducted at "[[physiological]] [[pH]]". |
||
| ⚫ | |||
Similar to Robinson synthesis, synthesis of [[Pseudopelletierine]] can be helpful for comparison. In tropinone synthesis [[succindialdehyde]] is used, while [[glutaraldehyde]] is used in the synthesis of Pseudopelletierine. |
|||
| ⚫ | |||
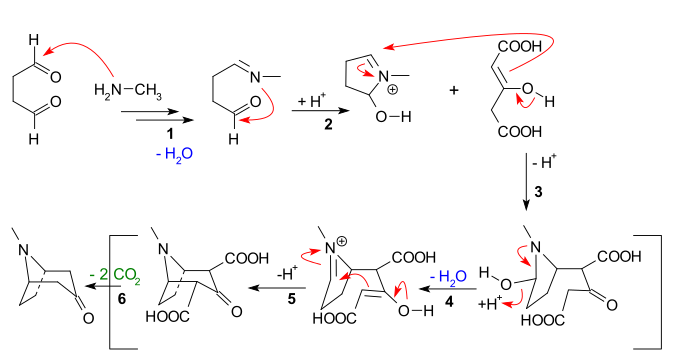
The main features apparent from the reaction sequence below are: |
The main features apparent from the reaction sequence below are: |
||
| Line 97: | Line 97: | ||
CO<sub>2</sub>R-tropinone has 4 stereoisomers, although the corresponding [[ecgonidine]] alkyl ester has only a pair of enantiomers. |
CO<sub>2</sub>R-tropinone has 4 stereoisomers, although the corresponding [[ecgonidine]] alkyl ester has only a pair of enantiomers. |
||
===Elming Modification=== |
|||
[[File:Elming Tropinone Modification.svg|thumb|center|500px|Elming Tropinone Modification: {{Cite patent|GB|791770}}]] |
|||
Elming et al. (1958) synthesized tropinone using [[methylamine hydrochloride]], [[acetonedicarboxylic acid]], and generating [[succindialdehyde]] in situ by the action of acid on [[2,5-dimethoxytetrahydrofuran]]. The yield was 81%, but in this case physiological conditions were not necessary. |
|||
== See also == |
== See also == |
||
Revision as of 08:45, 5 August 2015

| |

| |
| Names | |
|---|---|
| IUPAC name
8-Methyl-8-azabicyclo[3.2.1]octan-3-one
| |
| Other names
3-Tropinone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.756 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H13NO | |
| Molar mass | 139.195 g/mol |
| Appearance | Brown solid |
| Melting point | 42.5 °C (108.5 °F; 315.6 K) |
| Boiling point | (decomposes) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I.[1][2] Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure.
Synthesis
The first synthesis of tropinone was by Richard Willstätter in 1901. It started from the seemingly related cycloheptanone, but required many steps to introduce the nitrogen bridge; the overall yield for the synthesis path is only 0.75%.[3] Willstätter had previously synthesized cocaine from tropinone, in what was the first synthesis and elucidation of the structure of cocaine.[4]
The 1917 synthesis by Robinson is considered a classic in total synthesis[6] due to its simplicity and biomimetic approach. Tropinone is a bicyclic molecule, but the reactants used in its preparation are fairly simple: succinaldehyde, methylamine and acetonedicarboxylic acid (or even acetone). The synthesis is a good example of a biomimetic reaction or biogenetic-type synthesis because biosynthesis makes use of the same building blocks. It also demonstrates a tandem reaction in a one-pot synthesis. Furthermore the yield of the synthesis was 17% and with subsequent improvements exceeded 90%.[3]
This reaction is described as an intramolecular "double Mannich reaction" for obvious reasons. It is not unique in this regard, as others have also attempted it in piperidine synthesis.[7][8]
In place of acetone, acetonedicarboxylic acid is known as the "synthetic equivalent" the 1,3-dicarboxylic acid groups are so-called "activating groups" to facilitate the ring forming reactions. The calcium salt is there as a "buffer" as it is claimed that higher yields are possible if the reaction is conducted at "physiological pH".
Reaction mechanism
The main features apparent from the reaction sequence below are:
- Nucleophilic addition of methylamine to succinaldehyde, followed by loss of water to create an imine
- Intramolecular addition of the imine to the second aldehyde unit and first ring closure
- Intermolecular Mannich reaction of the enolate of acetone dicarboxylate
- New enolate formation and new imine formation with loss of water for
- Second intramolecular mannich reaction and second ring closure
- Loss of 2 carboxylic groups to tropinone
Some authors have actually tried to retain one of the CO2H groups.[9]
CO2R-tropinone has 4 stereoisomers, although the corresponding ecgonidine alkyl ester has only a pair of enantiomers.
See also
- 2-Carbomethoxytropinone (2-CMT) an intermediate in the creation of ecgonine cocaine analogues
References
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1039/CT9171100762, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1039/CT9171100762instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10649349, please use {{cite journal}} with
|pmid=10649349instead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1039/9781847551573, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1039/9781847551573instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1039/b001713m, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1039/b001713minstead. - ^ Doble, Mukesh; Kruthiventi, Anil Kumar (2007). Green Chemistry and Engineering. Oxford: Elsevier. p. 34. ISBN 978-0-12-372532-5.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1098/rsnr.1993.0034, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1098/rsnr.1993.0034instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10669562, please use {{cite journal}} with
|pmid=10669562instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11425577, please use {{cite journal}} with
|pmid=11425577instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jo01362a022, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jo01362a022instead.