Content deleted Content added
Ashleighhhhh (talk | contribs) mNo edit summary |
navbox |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
'''Naphthoylindoles''' are a class of [[synthetic cannabinoids]].<ref>{{cite journal | last1 = Manera | first1 = C | last2 = Tuccinardi | first2 = T | last3 = Martinelli | first3 = A | title = Indoles and related compounds as cannabinoid ligands | journal = Mini Reviews in Medicinal Chemistry | volume = 8 | issue = 4 | pages = 370–87 | year = 2008 | pmid = 18473928 |name-list-style=vanc | doi=10.2174/138955708783955935}}</ref> |
'''Naphthoylindoles''' are a class of [[synthetic cannabinoids]].<ref>{{cite journal | last1 = Manera | first1 = C | last2 = Tuccinardi | first2 = T | last3 = Martinelli | first3 = A | title = Indoles and related compounds as cannabinoid ligands | journal = Mini Reviews in Medicinal Chemistry | volume = 8 | issue = 4 | pages = 370–87 | year = 2008 | pmid = 18473928 |name-list-style=vanc | doi=10.2174/138955708783955935}}</ref> |
||
==Pharmacology== |
==Pharmacology== |
||
Behaving similarly [[in vivo]] to [[endocannabinoid]]s such as [[anandamide]] or [[2-arachidonoylglycerol]] (2-AG), Naphthoylindoles can bind to [[Endocannabinoid system|endocannabinoid]] [[Cannabinoid receptor|receptors]] in animals, presenting as CB<sub>1</sub> and/or CB<sub>2</sub> [[Partial agonist|partial]]/[[Full agonist|full]] [[agonist]]s. |
Behaving similarly [[in vivo]] to [[endocannabinoid]]s such as [[anandamide]] or [[2-arachidonoylglycerol]] (2-AG), Naphthoylindoles can bind to [[Endocannabinoid system|endocannabinoid]] [[Cannabinoid receptor|receptors]] in animals, presenting as [[Cannabinoid receptor 1|CB<sub>1</sub>]] and/or [[Cannabinoid receptor 2|CB<sub>2</sub>]] [[Partial agonist|partial]]/[[Full agonist|full]] [[agonist]]s. |
||
==History== |
==History== |
||
They have gained notoriety over the years for illicit usage and distribution in [[Europe]] and |
They have gained notoriety over the years for illicit usage and distribution in [[Europe]] and [[North America]], typically marketed as "herbal incense blends."<ref>{{cite web |title=Synthetic cannabinoids drug profile |url=https://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids_en |website=emcdda.europa.eu |publisher=EMCDDA |access-date=21 May 2023}}</ref> |
||
==See also== |
==See also== |
||
* [[Structural scheduling of synthetic cannabinoids]] |
* [[Structural scheduling of synthetic cannabinoids]] |
||
* [[List of JWH cannabinoids]], includes many naphthoylindoles |
|||
* [[Naphthoyl]], an [[acyl group]], derived, in this case, from [[1-naphthoic acid]] |
|||
* [[Indole]] |
|||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
{{Cannabinoids}} |
|||
[[Category:Naphthoylindoles| ]] |
[[Category:Naphthoylindoles| ]] |
||
Latest revision as of 20:54, 10 February 2024
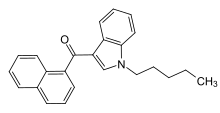
Naphthoylindoles are a class of synthetic cannabinoids.[1]
Pharmacology[edit]
Behaving similarly in vivo to endocannabinoids such as anandamide or 2-arachidonoylglycerol (2-AG), Naphthoylindoles can bind to endocannabinoid receptors in animals, presenting as CB1 and/or CB2 partial/full agonists.
History[edit]
They have gained notoriety over the years for illicit usage and distribution in Europe and North America, typically marketed as "herbal incense blends."[2]
See also[edit]
- Structural scheduling of synthetic cannabinoids
- List of JWH cannabinoids, includes many naphthoylindoles
- Naphthoyl, an acyl group, derived, in this case, from 1-naphthoic acid
- Indole
References[edit]
- ^ Manera C, Tuccinardi T, Martinelli A (2008). "Indoles and related compounds as cannabinoid ligands". Mini Reviews in Medicinal Chemistry. 8 (4): 370–87. doi:10.2174/138955708783955935. PMID 18473928.
- ^ "Synthetic cannabinoids drug profile". emcdda.europa.eu. EMCDDA. Retrieved 21 May 2023.