m →Enantiomers: Journal cites, templated 1 journal cites using AWB (11758) |
m →Enantiomers: Journal cites, Added 1 doi to a journal cite using AWB (11757) |
||
| Line 36: | Line 36: | ||
==Enantiomers== |
==Enantiomers== |
||
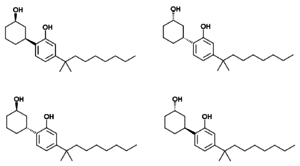
Cannabicyclohexanol has four [[enantiomer]]s, which by analogy with other related cannabinoid compounds can be expected to have widely varying affinity for cannabinoid receptors, and consequently will show considerable variation in potency.<ref>Eric Stern and Didier Lambert. Cannabinoids in Nature and Medicine. Medicinal Chemistry Endeavors around the Phytocannabinoids. pp 5, 130. ISBN 978-3-906390-56-7</ref> While the (-)-''cis'' enantiomer (-)-cannabicyclohexanol discovered in the original Pfizer research is expected to be the most potent, all four enantiomers have been isolated from illicit samples of this compound, and the properties of the other three enantiomers have not been studied in detail. Most commonly cannabicyclohexanol is encountered as a diastereomeric mix of the two ''cis'' or two ''trans'' isomers in varying ratios, though more rarely a mixture of all four enantiomers has been seen, as well as reasonably enantiopure samples of the most active isomer.<ref>{{cite journal | last1 = Uchiyama | first1 = N | last2 = Kikura-Hanajiri | first2 = R | last3 = Shoda | first3 = T | last4 = Fukuhara | first4 = K | last5 = Goda | first5 = Y | year = 2011 | title = Isomeric analysis of synthetic cannabinoids detected as designer drugs | url = | journal = Yakugaku Zasshi | volume = 131 | issue = 7| pages = 1141–7 | pmid = 21720146 }}</ref><ref>[http://www.emcdda.europa.eu/attachements.cfm/att_132911_EN_2009_Implementation report.pdf Europol 2009 Annual Report on the implementation of Council Decision 2005/387/JHA]</ref><ref>[http://www.emcdda.europa.eu/attachements.cfm/att_212366_EN_EMCDDA-Europol 2012 Annual Report_final.pdf Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA]</ref> Confusion can arise around the naming of these compounds as they can be viewed either as substituted [[phenol]]s or substituted [[cyclohexanol]]s, but this results in different numbering of the rings. Consequently the active isomer can be named either 2-[(1S,3R)-3-hydroxycyclohexyl]-5-(2-methylnonan-2-yl)phenol or (1R,3S)-3-[2-hydroxy-4-(2-methylnonan-2-yl)phenyl]cyclohexan-1-ol. |
Cannabicyclohexanol has four [[enantiomer]]s, which by analogy with other related cannabinoid compounds can be expected to have widely varying affinity for cannabinoid receptors, and consequently will show considerable variation in potency.<ref>Eric Stern and Didier Lambert. Cannabinoids in Nature and Medicine. Medicinal Chemistry Endeavors around the Phytocannabinoids. pp 5, 130. ISBN 978-3-906390-56-7</ref> While the (-)-''cis'' enantiomer (-)-cannabicyclohexanol discovered in the original Pfizer research is expected to be the most potent, all four enantiomers have been isolated from illicit samples of this compound, and the properties of the other three enantiomers have not been studied in detail. Most commonly cannabicyclohexanol is encountered as a diastereomeric mix of the two ''cis'' or two ''trans'' isomers in varying ratios, though more rarely a mixture of all four enantiomers has been seen, as well as reasonably enantiopure samples of the most active isomer.<ref>{{cite journal | last1 = Uchiyama | first1 = N | last2 = Kikura-Hanajiri | first2 = R | last3 = Shoda | first3 = T | last4 = Fukuhara | first4 = K | last5 = Goda | first5 = Y | year = 2011 | title = Isomeric analysis of synthetic cannabinoids detected as designer drugs | url = | journal = Yakugaku Zasshi | volume = 131 | issue = 7| pages = 1141–7 | pmid = 21720146 | doi=10.1248/yakushi.131.1141}}</ref><ref>[http://www.emcdda.europa.eu/attachements.cfm/att_132911_EN_2009_Implementation report.pdf Europol 2009 Annual Report on the implementation of Council Decision 2005/387/JHA]</ref><ref>[http://www.emcdda.europa.eu/attachements.cfm/att_212366_EN_EMCDDA-Europol 2012 Annual Report_final.pdf Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA]</ref> Confusion can arise around the naming of these compounds as they can be viewed either as substituted [[phenol]]s or substituted [[cyclohexanol]]s, but this results in different numbering of the rings. Consequently the active isomer can be named either 2-[(1S,3R)-3-hydroxycyclohexyl]-5-(2-methylnonan-2-yl)phenol or (1R,3S)-3-[2-hydroxy-4-(2-methylnonan-2-yl)phenyl]cyclohexan-1-ol. |
||
[[Image:Cannabicyclohexanol_isomers.png|300px|thumb|left|The four enantiomers of cannabicyclohexanol]]{{clear left}} |
[[Image:Cannabicyclohexanol_isomers.png|300px|thumb|left|The four enantiomers of cannabicyclohexanol]]{{clear left}} |
||
Revision as of 09:29, 19 December 2015
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.839 |
| Chemical and physical data | |
| Formula | C22H36O2 |
| Molar mass | 332.519 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
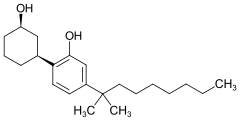
Cannabicyclohexanol (CCH, CP 47,497 dimethyloctyl homologue, (C8)-CP 47,497) is a cannabinoid receptor agonist drug, developed by Pfizer in 1979. On 19 January 2009, the University of Freiburg in Germany announced that an analog of CP 47,497 was the main active ingredient in the herbal incense product Spice, specifically the 1,1-dimethyloctyl homologue of CP 47,497, which is now known as cannabicyclohexanol.[2][3][4] The 1,1-dimethyloctyl homologue of CP 47,497 is in fact several times more potent than the parent compound,[5] which is somewhat unexpected as the 1,1-dimethylheptyl is the most potent substituent in classical cannabinoid compounds such as HU-210.[6]
Toxicity
(C8)-CP 47,497 has been shown to cause DNA damage and inflammation in directly exposed human cells in vitro,[7] though it is unclear if this has any relevance in vivo.
Enantiomers
Cannabicyclohexanol has four enantiomers, which by analogy with other related cannabinoid compounds can be expected to have widely varying affinity for cannabinoid receptors, and consequently will show considerable variation in potency.[8] While the (-)-cis enantiomer (-)-cannabicyclohexanol discovered in the original Pfizer research is expected to be the most potent, all four enantiomers have been isolated from illicit samples of this compound, and the properties of the other three enantiomers have not been studied in detail. Most commonly cannabicyclohexanol is encountered as a diastereomeric mix of the two cis or two trans isomers in varying ratios, though more rarely a mixture of all four enantiomers has been seen, as well as reasonably enantiopure samples of the most active isomer.[9][10][11] Confusion can arise around the naming of these compounds as they can be viewed either as substituted phenols or substituted cyclohexanols, but this results in different numbering of the rings. Consequently the active isomer can be named either 2-[(1S,3R)-3-hydroxycyclohexyl]-5-(2-methylnonan-2-yl)phenol or (1R,3S)-3-[2-hydroxy-4-(2-methylnonan-2-yl)phenyl]cyclohexan-1-ol.
See also
- Synthetic cannabis
- (C6)-CP 47,497
- (C7)-CP 47,497 (CP 47,497 itself)
- (C9)-CP 47,497
- O-1871
References
- ^ Cook, Morgan (28 February 2008). "Synthetic marijuana illegal as of Tuesday". The San Diego Union-Tribune. San Diego. Retrieved 25 July 2015.
- ^ "Hauptwirkstoff von "Spice" identifiziert". University of Freiburg. 19 March 2009. Retrieved 25 July 2015.
- ^ Volker Auwärter, Sebastian Dresen, Wolfgang Weinmann, Michael Müller, Michael Pütz, Nerea Ferreirós (May 2009). "'Spice' and other herbal blends: harmless incense or cannabinoid designer drugs?". Journal of Mass Spectrometry. 44 (5): 832–837. doi:10.1002/jms.1558. PMID 19189348.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y (May 2010). "Chemical analysis of synthetic cannabinoids as designer drugs in herbal products". Forensic Science International. 198 (1–3): 31–8. doi:10.1016/j.forsciint.2010.01.004. PMID 20117892.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ D. R. Compton, M. R. Johnson, L. S. Melvin, B. R. Martin (January 1992). "Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents". The Journal of pharmacology and experimental therapeutics. 260 (1): 201–209. PMID 1309872.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Martin, Billy R.; Compton, David R.; Thomas, Brian F.; Prescott, William R.; Little, Patrick J.; Razdan, Raj K.; Johnson, M.Ross; Melvin, Lawrence S.; Mechoulam, Raphael; Susan J., Ward (1991). "Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs". Pharmacology Biochemistry and Behavior. 40 (3): 471–478. doi:10.1016/0091-3057(91)90349-7. ISSN 0091-3057.
- ^ Andrea Bileck, Franziska Ferk, Halh Al-Serori, Verena J. Koller, Besnik Muqaku, Alexander Haslberger, Volker Auwärter, Christopher Gerner, Siegfried Knasmüller (July 2015). "Impact of a synthetic cannabinoid (CP-47,497-C8) on protein expression in human cells: evidence for induction of inflammation and DNA damage". Archives of Toxicology. doi:10.1007/s00204-015-1569-7. PMID 26194647.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eric Stern and Didier Lambert. Cannabinoids in Nature and Medicine. Medicinal Chemistry Endeavors around the Phytocannabinoids. pp 5, 130. ISBN 978-3-906390-56-7
- ^ Uchiyama, N; Kikura-Hanajiri, R; Shoda, T; Fukuhara, K; Goda, Y (2011). "Isomeric analysis of synthetic cannabinoids detected as designer drugs". Yakugaku Zasshi. 131 (7): 1141–7. doi:10.1248/yakushi.131.1141. PMID 21720146.
- ^ report.pdf Europol 2009 Annual Report on the implementation of Council Decision 2005/387/JHA
- ^ 2012 Annual Report_final.pdf Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA