Content deleted Content added
No edit summary |
auto mw |
||
| (45 intermediate revisions by 28 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
|||
| ⚫ | |||
| Watchedfields = changed |
|||
| ⚫ | |||
| verifiedrevid = 444674170 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| molecular_weight = 426.892 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Clinical data--> |
|||
| ⚫ | '''BML-190''' ('''Indomethacin morpholinylamide''') is a drug used in scientific research |
||
| tradename = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Pharmacokinetic data--> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Identifiers--> |
|||
| ⚫ | |||
| CAS_number_Ref = {{cascite|correct|??}} |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| ⚫ | |||
<!--Chemical data--> |
|||
| ⚫ | |||
| C=23 | H=23 | Cl=1 | N=2 | O=4 |
|||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
| ChemSpiderID = 2321 |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChI = 1S/C23H23ClN2O4/c1-15-19(14-22(27)25-9-11-30-12-10-25)20-13-18(29-2)7-8-21(20)26(15)23(28)16-3-5-17(24)6-4-16/h3-8,13H,9-12,14H2,1-2H3 |
|||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChIKey = BJSDNVVWJYDOLK-UHFFFAOYSA-N |
|||
| ⚫ | |||
| ⚫ | '''BML-190''' ('''Indomethacin morpholinylamide''') is a drug used in scientific research that acts as a selective [[cannabinoid receptor 2|CB<sub>2</sub>]] [[inverse agonist]].<ref>{{cite journal | vauthors = New DC, Wong YH | title = BML-190 and AM251 act as inverse agonists at the human cannabinoid CB2 receptor: signalling via cAMP and inositol phosphates | journal = FEBS Letters | volume = 536 | issue = 1–3 | pages = 157–60 | date = February 2003 | pmid = 12586356 | doi = 10.1016/S0014-5793(03)00048-6 | s2cid = 38569901 | doi-access = free }}</ref> BML-190 is structurally derived from the [[NSAID]] [[indomethacin]] but has a quite different biological activity.<ref>{{cite journal | vauthors = Klegeris A, Bissonnette CJ, McGeer PL | title = Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor | journal = British Journal of Pharmacology | volume = 139 | issue = 4 | pages = 775–86 | date = June 2003 | pmid = 12813001 | pmc = 1573900 | doi = 10.1038/sj.bjp.0705304 }}</ref> The activity produced by this compound is disputed, with some sources referring to it as a CB<sub>2</sub> [[agonist]] rather than an inverse agonist;<ref>{{cite journal | vauthors = Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M, Di Marzo V | title = Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation | journal = Endocrinology | volume = 141 | issue = 1 | pages = 118–26 | date = January 2000 | pmid = 10614630 | doi = 10.1210/endo.141.1.7239 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Scutt A, Williamson EM | s2cid = 23624771 | title = Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors | journal = Calcified Tissue International | volume = 80 | issue = 1 | pages = 50–9 | date = January 2007 | pmid = 17205329 | doi = 10.1007/s00223-006-0171-7 }}</ref> this may reflect an error in classification, or alternatively it may produce different effects in different tissues, and more research is required to resolve this dispute. |
||
| ⚫ | |||
| ⚫ | |||
{{Cannabinoids}} |
{{Cannabinoids}} |
||
[[Category:Cannabinoids]] |
[[Category:Cannabinoids]] |
||
[[Category:4-Morpholinyl compounds]] |
|||
[[Category:Indole ethers at the benzene ring]] |
|||
[[Category:Benzamides]] |
|||
[[Category:Chloroarenes]] |
|||
[[Category:Tryptamines]] |
|||
| ⚫ | |||
Latest revision as of 07:48, 25 March 2024
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
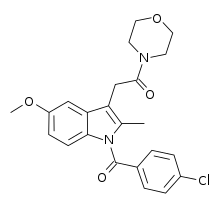
| Formula | C23H23ClN2O4 |
| Molar mass | 426.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
BML-190 (Indomethacin morpholinylamide) is a drug used in scientific research that acts as a selective CB2 inverse agonist.[1] BML-190 is structurally derived from the NSAID indomethacin but has a quite different biological activity.[2] The activity produced by this compound is disputed, with some sources referring to it as a CB2 agonist rather than an inverse agonist;[3][4] this may reflect an error in classification, or alternatively it may produce different effects in different tissues, and more research is required to resolve this dispute.
References[edit]
- ^ New DC, Wong YH (February 2003). "BML-190 and AM251 act as inverse agonists at the human cannabinoid CB2 receptor: signalling via cAMP and inositol phosphates". FEBS Letters. 536 (1–3): 157–60. doi:10.1016/S0014-5793(03)00048-6. PMID 12586356. S2CID 38569901.
- ^ Klegeris A, Bissonnette CJ, McGeer PL (June 2003). "Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor". British Journal of Pharmacology. 139 (4): 775–86. doi:10.1038/sj.bjp.0705304. PMC 1573900. PMID 12813001.
- ^ Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M, Di Marzo V (January 2000). "Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation". Endocrinology. 141 (1): 118–26. doi:10.1210/endo.141.1.7239. PMID 10614630.
- ^ Scutt A, Williamson EM (January 2007). "Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors". Calcified Tissue International. 80 (1): 50–9. doi:10.1007/s00223-006-0171-7. PMID 17205329. S2CID 23624771.