Content deleted Content added
BaeyerDrewson (talk | contribs) mNo edit summary |
m did general fixes if needed, replaced: See Also → See also |
||
| Line 8: | Line 8: | ||
| tradename = |
| tradename = |
||
| legal_status = |
| legal_status = |
||
| routes_of_administration = |
| routes_of_administration = |
||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| metabolism = |
| metabolism = |
||
| elimination_half-life = |
| elimination_half-life = |
||
| excretion = |
| excretion = |
||
<!--Identifiers--> |
<!--Identifiers--> |
||
| Line 35: | Line 35: | ||
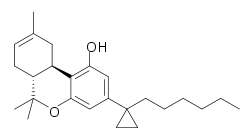
'''AMG-41''' (part of the [[List of AM cannabinoids|AM cannabinoid series]]) is an [[analgesic]] drug which is a [[cannabinoid]] [[agonist]]. It is a derivative of Δ8-[[THC]] substituted with a [[cyclopropyl group]] on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both [[Cannabinoid receptor 1|CB<sub>1</sub>]] and [[Cannabinoid receptor 2|CB<sub>2</sub>]], with a [[Dissociation constant|K<sub>i</sub>]] of 0.44 nM at CB<sub>1</sub> vs 0.86 nM at CB<sub>2</sub>.<ref>Papahatjis DP, Nikas SP, Andreou T, Makriyannis A. Novel 1',1'-chain substituted Delta(8)-tetrahydrocannabinols. ''Bioorganic and Medicinal Chemistry Letters''. 2002 Dec 16;12(24):3583-6. PMID 12443781</ref><ref>Papahatjis DP, ''et al.'' Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1'. ''Journal of Medicinal Chemistry''. 2003 Jul 17;46(15):3221-9. PMID 12852753</ref><ref>Papahatjis DP, ''et al.'' C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols. ''Journal of Medicinal Chemistry''. 2007 Aug 23;50(17):4048-60. PMID 17672444</ref> |
'''AMG-41''' (part of the [[List of AM cannabinoids|AM cannabinoid series]]) is an [[analgesic]] drug which is a [[cannabinoid]] [[agonist]]. It is a derivative of Δ8-[[THC]] substituted with a [[cyclopropyl group]] on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both [[Cannabinoid receptor 1|CB<sub>1</sub>]] and [[Cannabinoid receptor 2|CB<sub>2</sub>]], with a [[Dissociation constant|K<sub>i</sub>]] of 0.44 nM at CB<sub>1</sub> vs 0.86 nM at CB<sub>2</sub>.<ref>Papahatjis DP, Nikas SP, Andreou T, Makriyannis A. Novel 1',1'-chain substituted Delta(8)-tetrahydrocannabinols. ''Bioorganic and Medicinal Chemistry Letters''. 2002 Dec 16;12(24):3583-6. PMID 12443781</ref><ref>Papahatjis DP, ''et al.'' Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1'. ''Journal of Medicinal Chemistry''. 2003 Jul 17;46(15):3221-9. PMID 12852753</ref><ref>Papahatjis DP, ''et al.'' C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols. ''Journal of Medicinal Chemistry''. 2007 Aug 23;50(17):4048-60. PMID 17672444</ref> |
||
==See |
==See also== |
||
* [[AMG-3]] |
* [[AMG-3]] |
||
* [[AMG-36]] |
* [[AMG-36]] |
||
| Line 43: | Line 43: | ||
{{cannabinoids}} |
{{cannabinoids}} |
||
| ⚫ | |||
[[Category:Cannabinoids]] |
[[Category:Cannabinoids]] |
||
[[Category:Benzochromenes]] |
[[Category:Benzochromenes]] |
||
[[Category:Phenols]] |
[[Category:Phenols]] |
||
| ⚫ | |||
Revision as of 00:40, 30 July 2016
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H36O2 |
| Molar mass | 368.551 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
AMG-41 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with a cyclopropyl group on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both CB1 and CB2, with a Ki of 0.44 nM at CB1 vs 0.86 nM at CB2.[1][2][3]
See also
References
- ^ Papahatjis DP, Nikas SP, Andreou T, Makriyannis A. Novel 1',1'-chain substituted Delta(8)-tetrahydrocannabinols. Bioorganic and Medicinal Chemistry Letters. 2002 Dec 16;12(24):3583-6. PMID 12443781
- ^ Papahatjis DP, et al. Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1'. Journal of Medicinal Chemistry. 2003 Jul 17;46(15):3221-9. PMID 12852753
- ^ Papahatjis DP, et al. C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols. Journal of Medicinal Chemistry. 2007 Aug 23;50(17):4048-60. PMID 17672444