Content deleted Content added
No edit summary |
m Bot: Deprecating Template:Cite doi and some minor fixes |
||
| Line 34: | Line 34: | ||
}} |
}} |
||
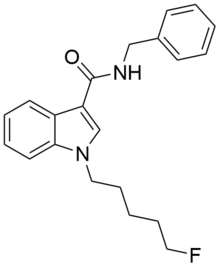
'''5F-SDB-006''' is a drug that acts as a potent [[agonist]] for the [[cannabinoid receptor]]s, with an EC<sub>50</sub> of 50 nM for human CB<sub>1</sub> receptors, and 123 nM for human CB<sub>2</sub> receptors.<ref>{{ |
'''5F-SDB-006''' is a drug that acts as a potent [[agonist]] for the [[cannabinoid receptor]]s, with an EC<sub>50</sub> of 50 nM for human CB<sub>1</sub> receptors, and 123 nM for human CB<sub>2</sub> receptors.<ref>{{Cite journal | doi = 10.1021/acschemneuro.5b00107| title = Effects of Bioisosteric Fluorine in Synthetic Cannabinoid Designer Drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135| journal = ACS Chemical Neuroscience| pages = 150508124201002| year = 2015| last1 = Banister | first1 = S. D. | last2 = Stuart | first2 = J. | last3 = Kevin | first3 = R. C. | last4 = Edington | first4 = A. | last5 = Longworth | first5 = M. | last6 = Wilkinson | first6 = S. M. | last7 = Beinat | first7 = C. | last8 = Buchanan | first8 = A. S. | last9 = Hibbs | first9 = D. E. | last10 = Glass | first10 = M. | last11 = Connor | first11 = M. | last12 = McGregor | first12 = I. S. | last13 = Kassiou | first13 = M. }}</ref> It was discovered during research into the related compound [[APICA]] which had been sold illicitly as "2NE1".<ref>{{Cite journal | last1 = Banister | first1 = S. D. | last2 = Wilkinson | first2 = S. M. | last3 = Longworth | first3 = M. | last4 = Stuart | first4 = J. | last5 = Apetz | first5 = N. | last6 = English | first6 = K. | last7 = Brooker | first7 = L. | last8 = Goebel | first8 = C. | last9 = Hibbs | first9 = D. E. | last10 = Glass | first10 = M. | last11 = Connor | first11 = M. | last12 = McGregor | first12 = I. S. | last13 = Kassiou | first13 = M. | title = The synthesis and pharmacological evaluation of adamantane-derived indoles: Novel cannabimimetic drugs of abuse | doi = 10.1021/cn400035r | journal = ACS Chemical Neuroscience | volume = 4 | issue = 7 | pages = 130403084729007 | year = 2013 | pmid = | pmc = }}</ref> 5F-SDB-006 is the terminally fluorinated analog of [[SDB-006]], just as [[STS-135 (drug)|STS-135]] is the terminally fluorinated analog of [[APICA (synthetic cannabinoid drug)|APICA]]. |
||
==See also== |
==See also== |
||
Revision as of 22:43, 29 August 2015
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H23FN2O |
| Molar mass | 338.43 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
5F-SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 50 nM for human CB1 receptors, and 123 nM for human CB2 receptors.[1] It was discovered during research into the related compound APICA which had been sold illicitly as "2NE1".[2] 5F-SDB-006 is the terminally fluorinated analog of SDB-006, just as STS-135 is the terminally fluorinated analog of APICA.
See also
References
- ^ Banister, S. D.; Stuart, J.; Kevin, R. C.; Edington, A.; Longworth, M.; Wilkinson, S. M.; Beinat, C.; Buchanan, A. S.; Hibbs, D. E.; Glass, M.; Connor, M.; McGregor, I. S.; Kassiou, M. (2015). "Effects of Bioisosteric Fluorine in Synthetic Cannabinoid Designer Drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience: 150508124201002. doi:10.1021/acschemneuro.5b00107.
- ^ Banister, S. D.; Wilkinson, S. M.; Longworth, M.; Stuart, J.; Apetz, N.; English, K.; Brooker, L.; Goebel, C.; Hibbs, D. E.; Glass, M.; Connor, M.; McGregor, I. S.; Kassiou, M. (2013). "The synthesis and pharmacological evaluation of adamantane-derived indoles: Novel cannabimimetic drugs of abuse". ACS Chemical Neuroscience. 4 (7): 130403084729007. doi:10.1021/cn400035r.