m formatting journal PMID cites using AWB (7391) |
Citation bot (talk | contribs) m Citations: [209] added: last1, first1, last2, first2, title, journal, volume, issue, pages, year, last3, first3, last4, first4, last5, first5, last6, first6, doi, last7, first7, last8, first8, last9, first9. Rjwilmsi |
||
| Line 25: | Line 25: | ||
'''11-''nor''-9-Carboxy-THC''', also known as '''11-''nor''-9-carboxy-delta-9-tetrahydrocannabinol''', '''11-COOH-THC''', '''THC-COOH''', and '''THC-11-oic acid''', is the main secondary [[metabolite]] of [[Tetrahydrocannabinol|THC]] which is formed in the body after [[Cannabis]] is consumed. |
'''11-''nor''-9-Carboxy-THC''', also known as '''11-''nor''-9-carboxy-delta-9-tetrahydrocannabinol''', '''11-COOH-THC''', '''THC-COOH''', and '''THC-11-oic acid''', is the main secondary [[metabolite]] of [[Tetrahydrocannabinol|THC]] which is formed in the body after [[Cannabis]] is consumed. |
||
11-COOH-THC is formed in the body by [[oxidation]] of the active metabolite [[11-Hydroxy-THC]] by liver enzymes. It is then metabolized further by conjugation with [[glucuronide]],<ref>{{cite journal | pmid = 11805011 }}</ref> forming a water-soluble congener which can be more easily excreted by the body.<ref>{{cite journal | pmid = 6323852 }}</ref> |
11-COOH-THC is formed in the body by [[oxidation]] of the active metabolite [[11-Hydroxy-THC]] by liver enzymes. It is then metabolized further by conjugation with [[glucuronide]],<ref>{{cite journal | last1 = Skopp | first1 = G | last2 = Pötsch | first2 = L | title = Stability of 11-nor-delta(9)-carboxy-tetrahydrocannabinol glucuronide in plasma and urine assessed by liquid chromatography-tandem mass spectrometry | journal = Clinical chemistry | volume = 48 | issue = 2 | pages = 301–6 | year = 2002 | pmid = 11805011 }}</ref> forming a water-soluble congener which can be more easily excreted by the body.<ref>{{cite journal | last1 = Law | first1 = B | last2 = Mason | first2 = PA | last3 = Moffat | first3 = AC | last4 = King | first4 = LJ | title = Confirmation of cannabis use by the analysis of delta 9-tetrahydrocannabinol metabolites in blood and urine by combined HPLC and RIA | journal = Journal of analytical toxicology | volume = 8 | issue = 1 | pages = 19–22 | year = 1984 | pmid = 6323852 }}</ref> |
||
11-COOH-THC is not [[psychoactive]] itself, but has a long half-life in the body of up to several days (or even weeks in very heavy users),<ref>{{cite journal | pmid = 8926739 }}</ref><ref>{{cite journal | pmid = 11576028 }}</ref><ref>{{cite journal | pmid = 17529896 }}</ref> making it the main metabolite tested for when [[Drug test|blood or urine testing]] for cannabis use. More sensitive tests are able to distinguish between 11-OH-THC and 11-COOH-THC, which can help determine how recently cannabis was consumed;<ref>{{cite journal | pmid = 1338216 }}</ref><ref>{{cite journal | pmid = 16885722 }}</ref> if only 11-COOH-THC is present then the cannabis was used some time ago and any impairment in cognitive ability or motor function will have dissipated, whereas if both 11-OH-THC and 11-COOH-THC are present then the cannabis was consumed more recently and motor impairment may still be present. |
11-COOH-THC is not [[psychoactive]] itself, but has a long half-life in the body of up to several days (or even weeks in very heavy users),<ref>{{cite journal | last1 = Huestis | first1 = MA | last2 = Mitchell | first2 = JM | last3 = Cone | first3 = EJ | title = Detection times of marijuana metabolites in urine by immunoassay and GC-MS | journal = Journal of analytical toxicology | volume = 19 | issue = 6 | pages = 443–9 | year = 1995 | pmid = 8926739 }}</ref><ref>{{cite journal | last1 = Pope Jr | first1 = HG | last2 = Gruber | first2 = AJ | last3 = Hudson | first3 = JI | last4 = Huestis | first4 = MA | last5 = Yurgelun-Todd | first5 = D | title = Neuropsychological performance in long-term cannabis users | journal = Archives of general psychiatry | volume = 58 | issue = 10 | pages = 909–15 | year = 2001 | pmid = 11576028 }}</ref><ref>{{cite journal | last1 = Dietz | first1 = L | last2 = Glaz-Sandberg | first2 = A | last3 = Nguyen | first3 = H | last4 = Skopp | first4 = G | last5 = Mikus | first5 = G | last6 = Aderjan | first6 = R | title = The urinary disposition of intravenously administered 11-nor-9-carboxy-delta-9-tetrahydrocannabinol in humans | journal = Therapeutic drug monitoring | volume = 29 | issue = 3 | pages = 368–72 | year = 2007 | pmid = 17529896 | doi = 10.1097/FTD.0b013e31805ba6fd }}</ref> making it the main metabolite tested for when [[Drug test|blood or urine testing]] for cannabis use. More sensitive tests are able to distinguish between 11-OH-THC and 11-COOH-THC, which can help determine how recently cannabis was consumed;<ref>{{cite journal | last1 = Huestis | first1 = MA | last2 = Henningfield | first2 = JE | last3 = Cone | first3 = EJ | title = Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH) | journal = Journal of analytical toxicology | volume = 16 | issue = 5 | pages = 283–90 | year = 1992 | pmid = 1338216 }}</ref><ref>{{cite journal | last1 = Huestis | first1 = MA | last2 = Elsohly | first2 = M | last3 = Nebro | first3 = W | last4 = Barnes | first4 = A | last5 = Gustafson | first5 = RA | last6 = Smith | first6 = ML | title = Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations | journal = Therapeutic drug monitoring | volume = 28 | issue = 4 | pages = 540–4 | year = 2006 | pmid = 16885722 }}</ref> if only 11-COOH-THC is present then the cannabis was used some time ago and any impairment in cognitive ability or motor function will have dissipated, whereas if both 11-OH-THC and 11-COOH-THC are present then the cannabis was consumed more recently and motor impairment may still be present. |
||
Some jurisdictions where cannabis use is decriminalized or permitted under some circumstances use such tests when determining whether drivers were [[Driving under the influence|legally intoxicated]] and therefore unfit to drive, with the comparative levels of THC, 11-OH-THC and 11-COOH-THC being used to derive a "blood cannabis level" analogous to the blood alcohol level used in prosecuting impaired drivers.<ref>{{cite journal | pmid = 16105257 }}</ref> On the other hand in jurisdictions where cannabis is completely illegal, any detectable levels of 11-COOH-THC may be deemed to constitute driving while intoxicated, even though this approach has been criticized as tantamount to prohibition of "driving whilst being a regular user of cannabis" regardless of the presence or absence of any actual impairment that might impact on driving performance. |
Some jurisdictions where cannabis use is decriminalized or permitted under some circumstances use such tests when determining whether drivers were [[Driving under the influence|legally intoxicated]] and therefore unfit to drive, with the comparative levels of THC, 11-OH-THC and 11-COOH-THC being used to derive a "blood cannabis level" analogous to the blood alcohol level used in prosecuting impaired drivers.<ref>{{cite journal | last1 = Ménétrey | first1 = A | last2 = Augsburger | first2 = M | last3 = Favrat | first3 = B | last4 = Pin | first4 = MA | last5 = Rothuizen | first5 = LE | last6 = Appenzeller | first6 = M | last7 = Buclin | first7 = T | last8 = Mangin | first8 = P | last9 = Giroud | first9 = C | title = Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoids levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Delta9-THC | journal = Journal of analytical toxicology | volume = 29 | issue = 5 | pages = 327–38 | year = 2005 | pmid = 16105257 }}</ref> On the other hand in jurisdictions where cannabis is completely illegal, any detectable levels of 11-COOH-THC may be deemed to constitute driving while intoxicated, even though this approach has been criticized as tantamount to prohibition of "driving whilst being a regular user of cannabis" regardless of the presence or absence of any actual impairment that might impact on driving performance. |
||
While 11-COOH-THC does not have any psychoactive effects in its own right, it may still have a role in the [[analgesic]] and [[antiinflammatory]] effects of cannabis,<ref>{{cite journal | pmid = 2846397 }}</ref><ref>{{cite journal | pmid = 2178317 }}</ref> and has also been shown to moderate the effects of THC itself which may help explain the difference in subjective effects seen between occasional and regular users of cannabis.<ref>{{cite journal | pmid = 3032669 }}</ref><ref>{{cite journal | pmid = 3017356 }}</ref> |
While 11-COOH-THC does not have any psychoactive effects in its own right, it may still have a role in the [[analgesic]] and [[antiinflammatory]] effects of cannabis,<ref>{{cite journal | last1 = Burstein | first1 = SH | last2 = Hull | first2 = K | last3 = Hunter | first3 = SA | last4 = Latham | first4 = V | title = Cannabinoids and pain responses: a possible role for prostaglandins | journal = The FASEB journal : official publication of the Federation of American Societies for Experimental Biology | volume = 2 | issue = 14 | pages = 3022–6 | year = 1988 | pmid = 2846397 }}</ref><ref>{{cite journal | last1 = Doyle | first1 = SA | last2 = Burstein | first2 = SH | last3 = Dewey | first3 = WL | last4 = Welch | first4 = SP | title = Further studies on the antinociceptive effects of delta 6-THC-7-oic acid | journal = Agents and actions | volume = 31 | issue = 1-2 | pages = 157–63 | year = 1990 | pmid = 2178317 }}</ref> and has also been shown to moderate the effects of THC itself which may help explain the difference in subjective effects seen between occasional and regular users of cannabis.<ref>{{cite journal | last1 = Burstein | first1 = S | last2 = Hunter | first2 = SA | last3 = Latham | first3 = V | last4 = Renzulli | first4 = L | title = A major metabolite of delta 1-tetrahydrocannabinol reduces its cataleptic effect in mice | journal = Experientia | volume = 43 | issue = 4 | pages = 402–3 | year = 1987 | pmid = 3032669 }}</ref><ref>{{cite journal | last1 = Burstein | first1 = S | last2 = Hunter | first2 = SA | last3 = Latham | first3 = V | last4 = Renzulli | first4 = L | title = Prostaglandins and cannabis--XVI. Antagonism of delta 1-tetrahydrocannabinol action by its metabolites | journal = Biochemical pharmacology | volume = 35 | issue = 15 | pages = 2553–8 | year = 1986 | pmid = 3017356 }}</ref> |
||
== References == |
== References == |
||
Revision as of 20:03, 14 November 2010
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Variable |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Variable |
| Metabolism | Variable |
| Elimination half-life | 30 to 120 days [citation needed] |
| Excretion | Variable |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
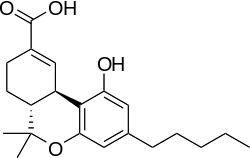
| Formula | C21H28O4 |
| Molar mass | 344.445 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
11-nor-9-Carboxy-THC, also known as 11-nor-9-carboxy-delta-9-tetrahydrocannabinol, 11-COOH-THC, THC-COOH, and THC-11-oic acid, is the main secondary metabolite of THC which is formed in the body after Cannabis is consumed.
11-COOH-THC is formed in the body by oxidation of the active metabolite 11-Hydroxy-THC by liver enzymes. It is then metabolized further by conjugation with glucuronide,[1] forming a water-soluble congener which can be more easily excreted by the body.[2]
11-COOH-THC is not psychoactive itself, but has a long half-life in the body of up to several days (or even weeks in very heavy users),[3][4][5] making it the main metabolite tested for when blood or urine testing for cannabis use. More sensitive tests are able to distinguish between 11-OH-THC and 11-COOH-THC, which can help determine how recently cannabis was consumed;[6][7] if only 11-COOH-THC is present then the cannabis was used some time ago and any impairment in cognitive ability or motor function will have dissipated, whereas if both 11-OH-THC and 11-COOH-THC are present then the cannabis was consumed more recently and motor impairment may still be present.
Some jurisdictions where cannabis use is decriminalized or permitted under some circumstances use such tests when determining whether drivers were legally intoxicated and therefore unfit to drive, with the comparative levels of THC, 11-OH-THC and 11-COOH-THC being used to derive a "blood cannabis level" analogous to the blood alcohol level used in prosecuting impaired drivers.[8] On the other hand in jurisdictions where cannabis is completely illegal, any detectable levels of 11-COOH-THC may be deemed to constitute driving while intoxicated, even though this approach has been criticized as tantamount to prohibition of "driving whilst being a regular user of cannabis" regardless of the presence or absence of any actual impairment that might impact on driving performance.
While 11-COOH-THC does not have any psychoactive effects in its own right, it may still have a role in the analgesic and antiinflammatory effects of cannabis,[9][10] and has also been shown to moderate the effects of THC itself which may help explain the difference in subjective effects seen between occasional and regular users of cannabis.[11][12]
References
- ^ Skopp, G; Pötsch, L (2002). "Stability of 11-nor-delta(9)-carboxy-tetrahydrocannabinol glucuronide in plasma and urine assessed by liquid chromatography-tandem mass spectrometry". Clinical chemistry. 48 (2): 301–6. PMID 11805011.
- ^ Law, B; Mason, PA; Moffat, AC; King, LJ (1984). "Confirmation of cannabis use by the analysis of delta 9-tetrahydrocannabinol metabolites in blood and urine by combined HPLC and RIA". Journal of analytical toxicology. 8 (1): 19–22. PMID 6323852.
- ^ Huestis, MA; Mitchell, JM; Cone, EJ (1995). "Detection times of marijuana metabolites in urine by immunoassay and GC-MS". Journal of analytical toxicology. 19 (6): 443–9. PMID 8926739.
- ^ Pope Jr, HG; Gruber, AJ; Hudson, JI; Huestis, MA; Yurgelun-Todd, D (2001). "Neuropsychological performance in long-term cannabis users". Archives of general psychiatry. 58 (10): 909–15. PMID 11576028.
- ^ Dietz, L; Glaz-Sandberg, A; Nguyen, H; Skopp, G; Mikus, G; Aderjan, R (2007). "The urinary disposition of intravenously administered 11-nor-9-carboxy-delta-9-tetrahydrocannabinol in humans". Therapeutic drug monitoring. 29 (3): 368–72. doi:10.1097/FTD.0b013e31805ba6fd. PMID 17529896.
- ^ Huestis, MA; Henningfield, JE; Cone, EJ (1992). "Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH)". Journal of analytical toxicology. 16 (5): 283–90. PMID 1338216.
- ^ Huestis, MA; Elsohly, M; Nebro, W; Barnes, A; Gustafson, RA; Smith, ML (2006). "Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations". Therapeutic drug monitoring. 28 (4): 540–4. PMID 16885722.
- ^ Ménétrey, A; Augsburger, M; Favrat, B; Pin, MA; Rothuizen, LE; Appenzeller, M; Buclin, T; Mangin, P; Giroud, C (2005). "Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoids levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Delta9-THC". Journal of analytical toxicology. 29 (5): 327–38. PMID 16105257.
- ^ Burstein, SH; Hull, K; Hunter, SA; Latham, V (1988). "Cannabinoids and pain responses: a possible role for prostaglandins". The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2 (14): 3022–6. PMID 2846397.
- ^ Doyle, SA; Burstein, SH; Dewey, WL; Welch, SP (1990). "Further studies on the antinociceptive effects of delta 6-THC-7-oic acid". Agents and actions. 31 (1–2): 157–63. PMID 2178317.
- ^ Burstein, S; Hunter, SA; Latham, V; Renzulli, L (1987). "A major metabolite of delta 1-tetrahydrocannabinol reduces its cataleptic effect in mice". Experientia. 43 (4): 402–3. PMID 3032669.
- ^ Burstein, S; Hunter, SA; Latham, V; Renzulli, L (1986). "Prostaglandins and cannabis--XVI. Antagonism of delta 1-tetrahydrocannabinol action by its metabolites". Biochemical pharmacology. 35 (15): 2553–8. PMID 3017356.