Flyer22 Frozen (talk | contribs) m Reverted unexplained removal of content (HG) |
Removed content because the research was insufficient. |
||
| Line 80: | Line 80: | ||
The effects of resveratrol are currently a topic of numerous animal and human studies. The mainstream press wrote about resveratrol's anti-aging effects,<ref>{{cite news| url=http://www.reuters.com/article/idUSTRE6350L620100406 | title=Pharma seeks genetic clues to healthy aging | date=6 April 2010 | work=Reuters}}</ref> but now, as of 19 December 2013 there are accepted data to form a scientific basis for the application of these claims to mammals.<ref>http://www.sciencedaily.com/releases/2013/12/131219130738.htm</ref> |
The effects of resveratrol are currently a topic of numerous animal and human studies. The mainstream press wrote about resveratrol's anti-aging effects,<ref>{{cite news| url=http://www.reuters.com/article/idUSTRE6350L620100406 | title=Pharma seeks genetic clues to healthy aging | date=6 April 2010 | work=Reuters}}</ref> but now, as of 19 December 2013 there are accepted data to form a scientific basis for the application of these claims to mammals.<ref>http://www.sciencedaily.com/releases/2013/12/131219130738.htm</ref> |
||
A series of early reports found that it increased the [[life expectancy|lifespan]] of model organisms. Several scientists involved in these studies went on to found [[Sirtris Pharmaceuticals]], a company working to develop resveratrol analogs as proprietary drugs. |
A series of early reports found that it increased the [[life expectancy|lifespan]] of model organisms. Several scientists involved in these studies went on to found [[Sirtris Pharmaceuticals]], a company working to develop resveratrol analogs as proprietary drugs. In mouse and rat experiments, [[telomere]] lengthening, [[telomerase]] activity enhancement, [[anti-inflammatory]], blood sugar-lowering and other beneficial [[cardiovascular]] effects of resveratrol have been reported.<ref name="Pearson2008">{{cite journal | author = Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R | title = Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span | journal = Cell Metab. | volume = 8 | issue = 2 | pages = 157–68 |date=August 2008 | pmid = 18599363 | pmc = 2538685 | doi = 10.1016/j.cmet.2008.06.011 }}</ref><ref name="Miller2010">{{cite journal | author = Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R | title = Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice | journal = J. Gerontol. A Biol. Sci. Med. Sci. | volume = 66 | issue = 2 | pages = 191–201 |date=February 2011 | pmid = 20974732 | pmc = 3021372 | doi = 10.1093/gerona/glq178 }}</ref><ref name=Strong2013>{{cite journal | author = Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE | title = Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice | journal = J. Gerontol. A Biol. Sci. Med. Sci. | volume = 68 | issue = 1 | pages = 6–16 |date=January 2013 | pmid = 22451473 | doi = 10.1093/gerona/gls070 | pmc = 3598361 }}</ref> |
||
Limited human clinical trials have been completed. While the reported effects are often positive, resveratrol may have lesser benefits in humans.<ref name="urlLinus Pauling Institute at Oregon State University">{{cite web | url = http://lpi.oregonstate.edu/infocenter/phytochemicals/resveratrol | title = Micronutrient Information Center: Resveratrol | author = | date = | publisher = Linus Pauling Institute at Oregon State University | accessdate = 2012-01-13 }}</ref> At present, research on resveratrol is still in its infancy and the long-term effects of supplementation in humans are not known.<ref name="The Connecticut Post 2009">{{cite news |work=The Connecticut Post |title=Selling resveratrol: Wonder drug or snake oil? |date=August 31, 2009 |first=Melissa |last=Healy |agency=The Los Angeles Times |url=http://www.ctpost.com/news/article/Selling-resveratrol-Wonder-drug-or-snake-oil-3464.php}}</ref><ref name="Gehm BD, McAndrews JM, Chien PY, Jameson JL 1997 14138–43">{{cite journal | author = Gehm BD, McAndrews JM, Chien PY, Jameson JL | title = Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 94 | issue = 25 | pages = 14138–43 |date=December 1997 | pmid = 9391166 | pmc = 28446 | doi = 10.1073/pnas.94.25.14138 }}</ref> |
Limited human clinical trials have been completed. While the reported effects are often positive, resveratrol may have lesser benefits in humans.<ref name="urlLinus Pauling Institute at Oregon State University">{{cite web | url = http://lpi.oregonstate.edu/infocenter/phytochemicals/resveratrol | title = Micronutrient Information Center: Resveratrol | author = | date = | publisher = Linus Pauling Institute at Oregon State University | accessdate = 2012-01-13 }}</ref> At present, research on resveratrol is still in its infancy and the long-term effects of supplementation in humans are not known.<ref name="The Connecticut Post 2009">{{cite news |work=The Connecticut Post |title=Selling resveratrol: Wonder drug or snake oil? |date=August 31, 2009 |first=Melissa |last=Healy |agency=The Los Angeles Times |url=http://www.ctpost.com/news/article/Selling-resveratrol-Wonder-drug-or-snake-oil-3464.php}}</ref><ref name="Gehm BD, McAndrews JM, Chien PY, Jameson JL 1997 14138–43">{{cite journal | author = Gehm BD, McAndrews JM, Chien PY, Jameson JL | title = Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 94 | issue = 25 | pages = 14138–43 |date=December 1997 | pmid = 9391166 | pmc = 28446 | doi = 10.1073/pnas.94.25.14138 }}</ref> |
||
| Line 102: | Line 102: | ||
Later the same year, Sinclair reported that resveratrol counteracted the detrimental effects of a high-fat diet in mice. The high-fat diet was compounded by adding hydrogenated [[coconut oil]] to the standard diet; it provided 60% of energy from fat, and the mice on it consumed about 30% more calories than the mice on standard diet and became obese and diabetic. Mice on the high-fat diet exhibited a high mortality rate compared to mice fed the standard diet; mice fed the high-fat diet plus 22 mg/kg resveratrol had a 30% lower risk of death than the mice on the high-fat diet alone, making their death rates similar to those on the standard diet. The supplement also partially corrected a subset of the abnormal [[gene expression]] profile and abnormal [[insulin]] and [[glucose]] metabolism. Resveratrol supplements did not change the levels of free fatty acids and cholesterol, however, which were much higher than in the mice on standard diet.<ref name=Baur2006>{{cite journal | author = Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA | title = Resveratrol improves health and survival of mice on a high-calorie diet | journal = Nature | volume = 444 | issue = 7117 | pages = 337–42 |date=November 2006 | pmid = 17086191 | doi = 10.1038/nature05354 }}</ref> |
Later the same year, Sinclair reported that resveratrol counteracted the detrimental effects of a high-fat diet in mice. The high-fat diet was compounded by adding hydrogenated [[coconut oil]] to the standard diet; it provided 60% of energy from fat, and the mice on it consumed about 30% more calories than the mice on standard diet and became obese and diabetic. Mice on the high-fat diet exhibited a high mortality rate compared to mice fed the standard diet; mice fed the high-fat diet plus 22 mg/kg resveratrol had a 30% lower risk of death than the mice on the high-fat diet alone, making their death rates similar to those on the standard diet. The supplement also partially corrected a subset of the abnormal [[gene expression]] profile and abnormal [[insulin]] and [[glucose]] metabolism. Resveratrol supplements did not change the levels of free fatty acids and cholesterol, however, which were much higher than in the mice on standard diet.<ref name=Baur2006>{{cite journal | author = Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA | title = Resveratrol improves health and survival of mice on a high-calorie diet | journal = Nature | volume = 444 | issue = 7117 | pages = 337–42 |date=November 2006 | pmid = 17086191 | doi = 10.1038/nature05354 }}</ref> |
||
Sinclair later reported that resveratrol treatment had a range of beneficial effects, but did not increase the longevity of nonobese, ''[[ad libitum]]''–fed (freely-feeding) mice when started midlife. |
|||
Sinclair later reported that resveratrol treatment had a range of beneficial effects, but did not increase the longevity of nonobese, ''[[ad libitum]]''–fed (freely-feeding) mice when started midlife.<ref name="Pearson2008" /> Later, the [[National Institute on Aging]]'s Interventions Testing Program (ITP) <ref name="pmid17578509">{{cite journal | author = Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R | title = An Aging Interventions Testing Program: study design and interim report | journal = Aging Cell | volume = 6 | issue = 4 | pages = 565–75 |date=August 2007 | pmid = 17578509 | doi = 10.1111/j.1474-9726.2007.00311.x }}</ref> also tested three different doses of resveratrol in mice on a normal diet beginning in young adulthood, and again found no effect on lifespan, even at doses roughly eight times higher than those that had normalized the lifespan of the high-fat-fed, obese mice in the earlier study.<ref name="Miller2010" /> In a later study, the ITP also found that resveratrol still did not extend lifespan when it was administered starting in young adulthood.<ref name=Strong2013 /> It also does not extend the lifespan of rats.<ref name=daLuz2112 /> |
|||
=== Exercise and metabolism === |
=== Exercise and metabolism === |
||
| Line 109: | Line 109: | ||
In a study of 123 [[Finnish people|Finnish]] adults, those born with certain increased variations of the SIRT1 gene had faster metabolisms, helping them to burn more energy, indicating the same pathway shown in the laboratory mice works in humans.<ref name="Lagouge_2006"/> A later short-term study from Auwerx's laboratory in obese humans (similar to the obese mice in Auwerx's 2006 study) also found that resveratrol supplementation activated SIRT1 in this population.<ref name=Timmers2011>{{cite journal | author = Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P | title = Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans | journal = Cell Metab. | volume = 14 | issue = 5 | pages = 612–22 |date=November 2011 | pmid = 22055504 | doi = 10.1016/j.cmet.2011.10.002 }}</ref> |
In a study of 123 [[Finnish people|Finnish]] adults, those born with certain increased variations of the SIRT1 gene had faster metabolisms, helping them to burn more energy, indicating the same pathway shown in the laboratory mice works in humans.<ref name="Lagouge_2006"/> A later short-term study from Auwerx's laboratory in obese humans (similar to the obese mice in Auwerx's 2006 study) also found that resveratrol supplementation activated SIRT1 in this population.<ref name=Timmers2011>{{cite journal | author = Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P | title = Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans | journal = Cell Metab. | volume = 14 | issue = 5 | pages = 612–22 |date=November 2011 | pmid = 22055504 | doi = 10.1016/j.cmet.2011.10.002 }}</ref> |
||
However, a subsequent study found that resveratrol supplementation actually interfered with the beneficial effects of exercise in humans: when two groups of older men were put on an exercise regimen, those that received resveratrol supplements enjoyed less reduction in LDL (“bad”) cholesterol and less reduction in mean arterial (blood) pressure than people who received a [[placebo]], and people who exercised and received the placebo also enjoyed reductions in plasma triglycerides, while those who took resveratrol did not, despite the exercise. Resveratrol users may also possibly have gotten less gain in other factors where there were nominal differences in the results that did not reach statistical significance.<ref name=Gliemann2013>{{cite journal | author = Gliemann L, Schmidt JF, Olesen J, Biensø RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y | title = Resveratrol Blunts the Positive Effects of Exercise Training on Cardiovascular Health in Aged Men | journal = J. Physiol. (Lond.) | volume = 591| issue = Pt 20| pages = 5047–5059|date=August 2013 | pmid = 23878368 | doi = 10.1113/jphysiol.2013.258061 }}</ref> |
|||
=== Cancer prevention === |
=== Cancer prevention === |
||
| Line 176: | Line 174: | ||
=== Sirtuin activation === |
=== Sirtuin activation === |
||
Some of the benefits demonstrated in previous studies were overstated,<ref>{{cite news |url=http://www.rawstory.com/rs/2011/09/21/longevity-gene-may-be-dead-end-study/ |title='Longevity gene' may be dead end: study |date=September 12, 2011 |agency=[[Agence France-Presse]] |publisher=[[The Raw Story]]}}</ref><ref name=Ledford_2011>{{cite journal | author = Ledford H | title = Longevity genes challenged. Do sirtuins really lengthen lifespan? | journal = Nature |date=September 2011 | doi = 10.1038/news.2011.549 }}</ref> however, this study was challenged immediately,<ref name="pmid21938026">{{cite journal | author = Viswanathan M, Guarente L | title = Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes | journal = Nature | volume = 477 | issue = 7365 | pages = E1–2 |date=September 2011 | pmid = 21938026 | doi = 10.1038/nature10440 }}</ref> and a few experiments were suggested to be of inferior quality.<ref name="pmid21938058">{{cite journal | author = Lombard DB, Pletcher SD, Cantó C, Auwerx J | title = Ageing: longevity hits a roadblock | journal = Nature | volume = 477 | issue = 7365 | pages = 410–1 |date=September 2011 | pmid = 21938058 | doi = 10.1038/477410a }}</ref> |
|||
There is an explicit link between resveratrol and [[sirtuins]]; specifically that SIRT1 could be directly activated through an [[Allosteric regulation|allosteric mechanism]] common to chemically diverse STACs, including resveratrol—in other words, that an anti-aging protein in humans could be activated by resveratrol, at least in vitro and under certain experimental conditions.<ref name="pmid23471411">{{cite journal | author = Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA | title = Evidence for a common mechanism of SIRT1 regulation by allosteric activators | journal = Science | volume = 339 | issue = 6124 | pages = 1216–9 |date=March 2013 | pmid = 23471411 | doi = 10.1126/science.1231097 | pmc = 3799917 }}</ref> |
There is an explicit link between resveratrol and [[sirtuins]]; specifically that SIRT1 could be directly activated through an [[Allosteric regulation|allosteric mechanism]] common to chemically diverse STACs, including resveratrol—in other words, that an anti-aging protein in humans could be activated by resveratrol, at least in vitro and under certain experimental conditions.<ref name="pmid23471411">{{cite journal | author = Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA | title = Evidence for a common mechanism of SIRT1 regulation by allosteric activators | journal = Science | volume = 339 | issue = 6124 | pages = 1216–9 |date=March 2013 | pmid = 23471411 | doi = 10.1126/science.1231097 | pmc = 3799917 }}</ref> |
||
| Line 190: | Line 186: | ||
Full formal pharmacokinetics of oral resveratrol 2000 mg twice daily in humans, studying interaction with concurrent ethanol, quercetin, and fat meal has been published.<ref name="pmid20528005">{{cite journal | author = la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron DW | title = Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects | journal = Clin Pharmacokinet | volume = 49 | issue = 7 | pages = 449–54 |date=July 2010 | pmid = 20528005 | doi = 10.2165/11531820-000000000-00000 }}</ref> Mean peak serum resveratrol concentration was 1274 ng/ml at steady-state, which was reduced 46% by a fat meal at dosing. There was no effect of concurrent oral quercetin or ethanol. Healthy volunteers had frequently reported minor diarrhea, and laboratory measures identified slight changes in liver function tests and in serum potassium. No adverse effect on renal function was identified, although only eight healthy adults were observed in the two-week study. |
Full formal pharmacokinetics of oral resveratrol 2000 mg twice daily in humans, studying interaction with concurrent ethanol, quercetin, and fat meal has been published.<ref name="pmid20528005">{{cite journal | author = la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron DW | title = Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects | journal = Clin Pharmacokinet | volume = 49 | issue = 7 | pages = 449–54 |date=July 2010 | pmid = 20528005 | doi = 10.2165/11531820-000000000-00000 }}</ref> Mean peak serum resveratrol concentration was 1274 ng/ml at steady-state, which was reduced 46% by a fat meal at dosing. There was no effect of concurrent oral quercetin or ethanol. Healthy volunteers had frequently reported minor diarrhea, and laboratory measures identified slight changes in liver function tests and in serum potassium. No adverse effect on renal function was identified, although only eight healthy adults were observed in the two-week study. |
||
In humans and rats less than 5% of the oral dose was observed as free resveratrol in blood plasma.<ref name="pmid17548692"/><ref name="pmid15779067"/><ref name="Walle" /><ref name="pmid12065739">{{cite journal | author = Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP | title = Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model | journal = J. Pharmacol. Exp. Ther. | volume = 302 | issue = 1 | pages = 369–73 |date=July 2002 | pmid = 12065739 | doi = 10.1124/jpet.102.033340 }}</ref><ref name="pmid16869992">{{cite journal | author = Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice-Evans C, Spencer JP | title = Distribution of [3H]trans-resveratrol in rat tissues following oral administration | journal = Br. J. Nutr. | volume = 96 | issue = 1 | pages = 62–70 |date=July 2006 | pmid = 16869992 | doi = 10.1079/BJN20061810 }}</ref> The most abundant resveratrol metabolites in humans, rats, and mice are [[trans-resveratrol-3-O-glucuronide]] and trans-resveratrol-3-sulfate.<ref name="pmid12523673">{{cite journal | author = Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB | title = Human, rat, and mouse metabolism of resveratrol | journal = Pharm. Res. | volume = 19 | issue = 12 | pages = 1907–14 |date=December 2002 | pmid = 12523673 | doi = 10.1023/A:1021414129280 }}</ref> Walle suggests sulfate conjugates are the primary source of activity,<ref name=Walle/> Wang et al. suggests the glucuronides,<ref name="pmid15349955">{{cite journal | author = Wang LX, Heredia A, Song H, Zhang Z, Yu B, Davis C, Redfield R | title = Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity | journal = J Pharm Sci | volume = 93 | issue = 10 | pages = 2448–57 |date=October 2004 | pmid = 15349955 | doi = 10.1002/jps.20156 }}</ref> and Boocock et al. also emphasized the need for further study of the effects of the [[metabolite]]s, including the possibility of deconjugation to free resveratrol inside cells. |
In humans and rats less than 5% of the oral dose was observed as free resveratrol in blood plasma.<ref name="pmid17548692"/><ref name="pmid15779067"/><ref name="Walle" /><ref name="pmid12065739">{{cite journal | author = Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP | title = Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model | journal = J. Pharmacol. Exp. Ther. | volume = 302 | issue = 1 | pages = 369–73 |date=July 2002 | pmid = 12065739 | doi = 10.1124/jpet.102.033340 }}</ref><ref name="pmid16869992">{{cite journal | author = Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice-Evans C, Spencer JP | title = Distribution of [3H]trans-resveratrol in rat tissues following oral administration | journal = Br. J. Nutr. | volume = 96 | issue = 1 | pages = 62–70 |date=July 2006 | pmid = 16869992 | doi = 10.1079/BJN20061810 }}</ref> The most abundant resveratrol metabolites in humans, rats, and mice are [[trans-resveratrol-3-O-glucuronide]] and trans-resveratrol-3-sulfate.<ref name="pmid12523673">{{cite journal | author = Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB | title = Human, rat, and mouse metabolism of resveratrol | journal = Pharm. Res. | volume = 19 | issue = 12 | pages = 1907–14 |date=December 2002 | pmid = 12523673 | doi = 10.1023/A:1021414129280 }}</ref> Walle suggests sulfate conjugates are the primary source of activity,<ref name=Walle/> Wang et al. suggests the glucuronides,<ref name="pmid15349955">{{cite journal | author = Wang LX, Heredia A, Song H, Zhang Z, Yu B, Davis C, Redfield R | title = Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity | journal = J Pharm Sci | volume = 93 | issue = 10 | pages = 2448–57 |date=October 2004 | pmid = 15349955 | doi = 10.1002/jps.20156 }}</ref> and Boocock et al. also emphasized the need for further study of the effects of the [[metabolite]]s, including the possibility of deconjugation to free resveratrol inside cells. |
||
The hypothesis that resveratrol from wine could have higher bioavailability than resveratrol from a pill <ref name="pmid16732220"/><ref name="pmid15779070">{{cite journal | author = Wenzel E, Somoza V | title = Metabolism and bioavailability of trans-resveratrol | journal = Mol Nutr Food Res | volume = 49 | issue = 5 | pages = 472–81 |date=May 2005 | pmid = 15779070 | doi = 10.1002/mnfr.200500010 }}</ref> has been refuted by experimental data.<ref name="pmid12554065">{{cite journal | author = Goldberg DM, Yan J, Soleas GJ | title = Absorption of three wine-related polyphenols in three different matrices by healthy subjects | journal = Clin. Biochem. | volume = 36 | issue = 1 | pages = 79–87 |date=February 2003 | pmid = 12554065 | doi = 10.1016/S0009-9120(02)00397-1 }}</ref><ref name="pmid15830336">{{cite journal | author = Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi PP, Fogliano V, Marchelli R | title = Bioavailability of trans-resveratrol from red wine in humans | journal = Mol Nutr Food Res | volume = 49 | issue = 5 | pages = 495–504 |date=May 2005 | pmid = 15830336 | doi = 10.1002/mnfr.200500002 }}</ref> For example, after five men took 600 ml of red wine with the resveratrol content of 3.2 mg/l (total dose about 2 mg) before breakfast, unchanged resveratrol was detected in the blood of only two of them, and only in trace amounts (below 2.5 ng/ml). Resveratrol levels appeared to be slightly higher if red wine (600 ml of red wine containing 0.6 mg/ml resveratrol; total dose about 0.5 mg) was taken with a meal: trace amounts (1–6 ng/ml) were found in four out of ten subjects.<ref name="pmid15830336"/> In another study, the pharmacokinetics of resveratrol (25 mg) did not change whether it was taken with vegetable juice, white wine, or white grape juice. The highest level of unchanged resveratrol in the [[Serous fluid|serum]] (7–9 ng/ml) was achieved after 30 minutes, and it completely disappeared from blood after four hours.<ref name="pmid12554065"/> The authors of both studies concluded the trace amounts of resveratrol reached in the blood are insufficient to explain the French paradox. The beneficial effects of wine apparently could be explained by the effects of alcohol <ref name="pmid12554065"/> or the whole complex of substances wine contains;<ref name="pmid15830336"/> for example, the cardiovascular benefits of wine appear to correlate with the content of [[Proanthocyanidin|procyanidins]].<ref name="pmid17136085">{{cite journal | author = Corder R, Mullen W, Khan NQ, Marks SC, Wood EG, Carrier MJ, Crozier A | title = Oenology: red wine procyanidins and vascular health | journal = Nature | volume = 444 | issue = 7119 | pages = 566 |date=November 2006 | pmid = 17136085 | doi = 10.1038/444566a }}</ref> |
The hypothesis that resveratrol from wine could have higher bioavailability than resveratrol from a pill <ref name="pmid16732220"/><ref name="pmid15779070">{{cite journal | author = Wenzel E, Somoza V | title = Metabolism and bioavailability of trans-resveratrol | journal = Mol Nutr Food Res | volume = 49 | issue = 5 | pages = 472–81 |date=May 2005 | pmid = 15779070 | doi = 10.1002/mnfr.200500010 }}</ref> has been refuted by experimental data.<ref name="pmid12554065">{{cite journal | author = Goldberg DM, Yan J, Soleas GJ | title = Absorption of three wine-related polyphenols in three different matrices by healthy subjects | journal = Clin. Biochem. | volume = 36 | issue = 1 | pages = 79–87 |date=February 2003 | pmid = 12554065 | doi = 10.1016/S0009-9120(02)00397-1 }}</ref><ref name="pmid15830336">{{cite journal | author = Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi PP, Fogliano V, Marchelli R | title = Bioavailability of trans-resveratrol from red wine in humans | journal = Mol Nutr Food Res | volume = 49 | issue = 5 | pages = 495–504 |date=May 2005 | pmid = 15830336 | doi = 10.1002/mnfr.200500002 }}</ref> For example, after five men took 600 ml of red wine with the resveratrol content of 3.2 mg/l (total dose about 2 mg) before breakfast, unchanged resveratrol was detected in the blood of only two of them, and only in trace amounts (below 2.5 ng/ml). Resveratrol levels appeared to be slightly higher if red wine (600 ml of red wine containing 0.6 mg/ml resveratrol; total dose about 0.5 mg) was taken with a meal: trace amounts (1–6 ng/ml) were found in four out of ten subjects.<ref name="pmid15830336"/> In another study, the pharmacokinetics of resveratrol (25 mg) did not change whether it was taken with vegetable juice, white wine, or white grape juice. The highest level of unchanged resveratrol in the [[Serous fluid|serum]] (7–9 ng/ml) was achieved after 30 minutes, and it completely disappeared from blood after four hours.<ref name="pmid12554065"/> The authors of both studies concluded the trace amounts of resveratrol reached in the blood are insufficient to explain the French paradox. The beneficial effects of wine apparently could be explained by the effects of alcohol <ref name="pmid12554065"/> or the whole complex of substances wine contains;<ref name="pmid15830336"/> for example, the cardiovascular benefits of wine appear to correlate with the content of [[Proanthocyanidin|procyanidins]].<ref name="pmid17136085">{{cite journal | author = Corder R, Mullen W, Khan NQ, Marks SC, Wood EG, Carrier MJ, Crozier A | title = Oenology: red wine procyanidins and vascular health | journal = Nature | volume = 444 | issue = 7119 | pages = 566 |date=November 2006 | pmid = 17136085 | doi = 10.1038/444566a }}</ref> |
||
| Line 206: | Line 201: | ||
<div class="dablink">See [[:Calorie restriction#Sir2.2FSIRT1 and resveratrol]] for the details of the debate on resveratrol, calorie restriction and life extension.</div> |
<div class="dablink">See [[:Calorie restriction#Sir2.2FSIRT1 and resveratrol]] for the details of the debate on resveratrol, calorie restriction and life extension.</div> |
||
The mechanisms of resveratrol's apparent effects on life extension are not fully understood, but they appear to [[Caloric restriction mimetic|mimic]] several of the [[biochemical]] effects of [[calorie restriction]]. Some studies indicates resveratrol activates [[Sirtuin 1]]<ref>{{cite journal | author = Alcaín FJ, Villalba JM | title = Sirtuin activators | journal = Expert Opin Ther Pat | volume = 19 | issue = 4 | pages = 403–14 |date=April 2009 | pmid = 19441923 | doi = 10.1517/13543770902762893 }}</ref> and PGC-1α and improves the functioning of the [[mitochondria]].<ref name="Lagouge_2006"/> |
The mechanisms of resveratrol's apparent effects on life extension are not fully understood, but they appear to [[Caloric restriction mimetic|mimic]] several of the [[biochemical]] effects of [[calorie restriction]]. Some studies indicates resveratrol activates [[Sirtuin 1]]<ref>{{cite journal | author = Alcaín FJ, Villalba JM | title = Sirtuin activators | journal = Expert Opin Ther Pat | volume = 19 | issue = 4 | pages = 403–14 |date=April 2009 | pmid = 19441923 | doi = 10.1517/13543770902762893 }}</ref> and PGC-1α and improves the functioning of the [[mitochondria]].<ref name="Lagouge_2006"/> |
||
In cells treated with resveratrol, a fourteen-fold increase in the action of MnSOD ([[SOD2]]) is observed.<ref name="pmid18167310">{{cite journal | author = Robb EL, Page MM, Wiens BE, Stuart JA | title = Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD | journal = Biochem. Biophys. Res. Commun. | volume = 367 | issue = 2 | pages = 406–12 |date=March 2008 | pmid = 18167310 | doi = 10.1016/j.bbrc.2007.12.138 }}</ref> MnSOD reduces [[superoxide]] to [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>), but H<sub>2</sub>O<sub>2</sub> is not increased due to other cellular activity. Superoxide O<sub>2</sub><sup>−</sup> is a byproduct of respiration in complexes 1 and 3 of the [[electron transport chain]]. It is "not highly toxic, [but] can extract an electron from biological membrane and other cell components, causing free radical chain reactions. Therefore it is essential for the cell to keep superoxide anions in check."<ref>{{cite book |first=Zsolt |last=Radák |title=Free radicals in exercise and aging |publisher=Human Kinetics |location=Champaign, IL |year=2000 |page=39 |isbn=978-0-88011-881-1 |url=http://books.google.com/books?id=3TVElvqqR5EC&pg=PA39}}</ref> MnSOD reduces superoxide and thereby, confers resistance to [[Mitochondrial disease|mitochondrial dysfunction]], permeability transition, and apoptotic death in various diseases.<ref name="pmid11328670">{{cite journal | author = Macmillan-Crow LA, Cruthirds DL | title = Invited review: manganese superoxide dismutase in disease | journal = Free Radic. Res. | volume = 34 | issue = 4 | pages = 325–36 |date=April 2001 | pmid = 11328670 | doi = 10.1080/10715760100300281 }}</ref> It has been implicated in lifespan extension, inhibits cancer, (e.g. pancreatic cancer) <ref name="pmid12649190">{{cite journal | author = Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, Oberley LW | title = The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma | journal = Cancer Res. | volume = 63 | issue = 6 | pages = 1297–303 |date=March 2003 | pmid = 12649190 | doi = }}</ref><ref>{{cite press release |title=Mounting evidence shows red wine antioxidant kills cancer |publisher=[[University of Rochester Medical Center]] |date=March 26, 2008 |url=http://www.urmc.rochester.edu/news/story/index.cfm?id=1934 |accessdate=August 10, 2010}}</ref> and provides resistance to [[reperfusion injury]] and irradiation damage.<ref name="pmid12072463">{{cite journal | author = Sun J, Folk D, Bradley TJ, Tower J | title = Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster | journal = Genetics | volume = 161 | issue = 2 | pages = 661–72 |date=June 2002 | pmid = 12072463 | pmc = 1462135 | doi = }}</ref><ref name="pmid17129739">{{cite journal | author = Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E | title = Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase | journal = Neurobiol Learn Mem | volume = 87 | issue = 3 | pages = 372–84 |date=March 2007 | pmid = 17129739 | pmc = 1847321 | doi = 10.1016/j.nlm.2006.10.003 }}</ref><ref name="pmid7599209">{{cite journal | author = Wong GH | title = Protective roles of cytokines against radiation: induction of mitochondrial MnSOD | journal = Biochim. Biophys. Acta | volume = 1271 | issue = 1 | pages = 205–9 |date=May 1995 | pmid = 7599209 | doi =10.1016/0925-4439(95)00029-4 }}</ref> These effects have also been observed with resveratrol. Robb et al. propose MnSOD is increased by the pathway RESV → SIRT1 / NAD+ → FOXO3a → MnSOD. Resveratrol has been shown to cause SIRT1 to cause migration of FOXO transcription factors to the nucleus,<ref name="pmid17804521">{{cite journal | author = Stefani M, Markus MA, Lin RC, Pinese M, Dawes IW, Morris BJ | title = The effect of resveratrol on a cell model of human aging | journal = Annals of the New York Academy of Sciences | volume = 1114 | issue = | pages = 407–18 |date=October 2007 | pmid = 17804521 | doi = 10.1196/annals.1396.001 }}</ref> which stimulates FOXO3a transcriptional activity <ref name="pmid14976264">{{cite journal | author = Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME | title = Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase | journal = Science | volume = 303 | issue = 5666 | pages = 2011–5 |date=March 2004 | pmid = 14976264 | doi = 10.1126/science.1094637 }}</ref> and it has been shown to enhance the sirtuin-catalyzed deacetylation (activity) of [[FOXO3A|FOXO3a]]. MnSOD is known to be a target of FOXO3a, and MnSOD expression is strongly induced in cells overexpressing FOXO3a.<ref name="pmid12239572">{{cite journal | author = Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM | title = Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress | journal = Nature | volume = 419 | issue = 6904 | pages = 316–21 |date=September 2002 | pmid = 12239572 | doi = 10.1038/nature01036 }}</ref> It has been reported too that the disproportional up-regulation of superoxide dismutase (SOD), catalse (CAT) and glutathion peroxidase (GPX) expression (high expression of MnSOD, but mild change in CAT or GPX) and their enzymatic activity in cancer cells results in the mitochondrial accumulation of H2O2, which in turn induces cancer cell apoptosis.<ref name="pmid23456297">{{cite journal | author = Khan MA, Chen HC, Wan XX, Tania M, Xu AH, Chen FZ, Zhang DZ| title = Regulatory effects of resveratrol on antioxidant enzymes: a mechanism of growth inhibition and apoptosis induction in cancer cells | journal = Mol Cells | volume = 35 | issue = 3 | pages = 219–25 |date=March 2013 | pmid = 23456297 | doi = 10.1007/s10059-013-2259-z }}</ref> |
In cells treated with resveratrol, a fourteen-fold increase in the action of MnSOD ([[SOD2]]) is observed.<ref name="pmid18167310">{{cite journal | author = Robb EL, Page MM, Wiens BE, Stuart JA | title = Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD | journal = Biochem. Biophys. Res. Commun. | volume = 367 | issue = 2 | pages = 406–12 |date=March 2008 | pmid = 18167310 | doi = 10.1016/j.bbrc.2007.12.138 }}</ref> MnSOD reduces [[superoxide]] to [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>), but H<sub>2</sub>O<sub>2</sub> is not increased due to other cellular activity. Superoxide O<sub>2</sub><sup>−</sup> is a byproduct of respiration in complexes 1 and 3 of the [[electron transport chain]]. It is "not highly toxic, [but] can extract an electron from biological membrane and other cell components, causing free radical chain reactions. Therefore it is essential for the cell to keep superoxide anions in check."<ref>{{cite book |first=Zsolt |last=Radák |title=Free radicals in exercise and aging |publisher=Human Kinetics |location=Champaign, IL |year=2000 |page=39 |isbn=978-0-88011-881-1 |url=http://books.google.com/books?id=3TVElvqqR5EC&pg=PA39}}</ref> MnSOD reduces superoxide and thereby, confers resistance to [[Mitochondrial disease|mitochondrial dysfunction]], permeability transition, and apoptotic death in various diseases.<ref name="pmid11328670">{{cite journal | author = Macmillan-Crow LA, Cruthirds DL | title = Invited review: manganese superoxide dismutase in disease | journal = Free Radic. Res. | volume = 34 | issue = 4 | pages = 325–36 |date=April 2001 | pmid = 11328670 | doi = 10.1080/10715760100300281 }}</ref> It has been implicated in lifespan extension, inhibits cancer, (e.g. pancreatic cancer) <ref name="pmid12649190">{{cite journal | author = Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, Oberley LW | title = The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma | journal = Cancer Res. | volume = 63 | issue = 6 | pages = 1297–303 |date=March 2003 | pmid = 12649190 | doi = }}</ref><ref>{{cite press release |title=Mounting evidence shows red wine antioxidant kills cancer |publisher=[[University of Rochester Medical Center]] |date=March 26, 2008 |url=http://www.urmc.rochester.edu/news/story/index.cfm?id=1934 |accessdate=August 10, 2010}}</ref> and provides resistance to [[reperfusion injury]] and irradiation damage.<ref name="pmid12072463">{{cite journal | author = Sun J, Folk D, Bradley TJ, Tower J | title = Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster | journal = Genetics | volume = 161 | issue = 2 | pages = 661–72 |date=June 2002 | pmid = 12072463 | pmc = 1462135 | doi = }}</ref><ref name="pmid17129739">{{cite journal | author = Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E | title = Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase | journal = Neurobiol Learn Mem | volume = 87 | issue = 3 | pages = 372–84 |date=March 2007 | pmid = 17129739 | pmc = 1847321 | doi = 10.1016/j.nlm.2006.10.003 }}</ref><ref name="pmid7599209">{{cite journal | author = Wong GH | title = Protective roles of cytokines against radiation: induction of mitochondrial MnSOD | journal = Biochim. Biophys. Acta | volume = 1271 | issue = 1 | pages = 205–9 |date=May 1995 | pmid = 7599209 | doi =10.1016/0925-4439(95)00029-4 }}</ref> These effects have also been observed with resveratrol. Robb et al. propose MnSOD is increased by the pathway RESV → SIRT1 / NAD+ → FOXO3a → MnSOD. Resveratrol has been shown to cause SIRT1 to cause migration of FOXO transcription factors to the nucleus,<ref name="pmid17804521">{{cite journal | author = Stefani M, Markus MA, Lin RC, Pinese M, Dawes IW, Morris BJ | title = The effect of resveratrol on a cell model of human aging | journal = Annals of the New York Academy of Sciences | volume = 1114 | issue = | pages = 407–18 |date=October 2007 | pmid = 17804521 | doi = 10.1196/annals.1396.001 }}</ref> which stimulates FOXO3a transcriptional activity <ref name="pmid14976264">{{cite journal | author = Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME | title = Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase | journal = Science | volume = 303 | issue = 5666 | pages = 2011–5 |date=March 2004 | pmid = 14976264 | doi = 10.1126/science.1094637 }}</ref> and it has been shown to enhance the sirtuin-catalyzed deacetylation (activity) of [[FOXO3A|FOXO3a]]. MnSOD is known to be a target of FOXO3a, and MnSOD expression is strongly induced in cells overexpressing FOXO3a.<ref name="pmid12239572">{{cite journal | author = Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM | title = Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress | journal = Nature | volume = 419 | issue = 6904 | pages = 316–21 |date=September 2002 | pmid = 12239572 | doi = 10.1038/nature01036 }}</ref> It has been reported too that the disproportional up-regulation of superoxide dismutase (SOD), catalse (CAT) and glutathion peroxidase (GPX) expression (high expression of MnSOD, but mild change in CAT or GPX) and their enzymatic activity in cancer cells results in the mitochondrial accumulation of H2O2, which in turn induces cancer cell apoptosis.<ref name="pmid23456297">{{cite journal | author = Khan MA, Chen HC, Wan XX, Tania M, Xu AH, Chen FZ, Zhang DZ| title = Regulatory effects of resveratrol on antioxidant enzymes: a mechanism of growth inhibition and apoptosis induction in cancer cells | journal = Mol Cells | volume = 35 | issue = 3 | pages = 219–25 |date=March 2013 | pmid = 23456297 | doi = 10.1007/s10059-013-2259-z }}</ref> |
||
Revision as of 18:11, 12 February 2014

| |
| |
 Chemical structures of cis- ((Z)-resveratrol, left) and trans-resveratrol ((E)-resveratrol, right)[1]
| |
| Names | |
|---|---|
| Other names
trans-3,5,4'-Trihydroxystilbene;
3,4',5-Stilbenetriol; trans-Resveratrol; (E)-5-(p-Hydroxystyryl)resorcinol; (E)-5-(4-hydroxystyryl)benzene-1,3-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.121.386 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | white powder with slight yellow cast |
| Melting point | 261 - 263°C / 501.8 - 505.4°F[2] |
| Solubility in water | 0.03 g/L |
| Solubility in DMSO | 16 g/L |
| Solubility in ethanol | 50 g/L |
| UV-vis (λmax) | 304nm (trans-resveratrol, in water) 286nm (cis-resveratrol, in water)[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
23.2 µM (5,29 g)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Resveratrol (3,5,4'-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced naturally by several plants – especially the roots of the Japanese Knotweed, from which it is extracted commercially – when under attack by pathogens such as bacteria or fungi.
The effects of resveratrol are currently a topic of numerous animal and human studies. The mainstream press wrote about resveratrol's anti-aging effects,[5] but now, as of 19 December 2013 there are accepted data to form a scientific basis for the application of these claims to mammals.[6]
A series of early reports found that it increased the lifespan of model organisms. Several scientists involved in these studies went on to found Sirtris Pharmaceuticals, a company working to develop resveratrol analogs as proprietary drugs. In mouse and rat experiments, telomere lengthening, telomerase activity enhancement, anti-inflammatory, blood sugar-lowering and other beneficial cardiovascular effects of resveratrol have been reported.[7][8][9]
Limited human clinical trials have been completed. While the reported effects are often positive, resveratrol may have lesser benefits in humans.[10] At present, research on resveratrol is still in its infancy and the long-term effects of supplementation in humans are not known.[11][12]
Natural occurrence
Resveratrol is found in the skin of red grapes and in other fruits as well as in the roots of Japanese knotweed (Polygonum cuspidatum). Red wine contains on the order of 0.1-14.3 mg/l.[13] Resveratrol also has been produced by chemical synthesis [14] and by biotechnological synthesis (metabolic engineered microorganisms),[15][16] and it is sold as a nutritional supplement derived primarily from Japanese knotweed.[citation needed]
Resveratrol is also found in Gnetum cleistostachyum.[17]
Discovery and name
The first mention of resveratrol was in a Japanese article in 1939 by Michio Takaoka, who isolated it from the poisonous, but medicinal, Veratrum album, variety grandiflorum.[18] The name presumably comes from the fact that it is a resorcinol derivative coming from a Veratrum species.[19]
Scientific studies
Life extension
The groups of Howitz and David Sinclair (founder of Sirtris Pharmaceuticals) reported in 2003 that resveratrol significantly extends the lifespan of the yeast Saccharomyces cerevisiae.[20] Sinclair later reported that resveratrol also prolongs the lifespan of the worm, Caenorhabditis elegans, and the fruit fly, Drosophila melanogaster.[21] In 2007, a different group of researchers were able to reproduce Sinclair's results with C. elegans,[22] but other groups could not achieve consistent increases in lifespan of D. melanogaster, other flies, or C. elegans.[23][24][25]
First demonstration of life extension by resveratrol supplementation in a vertebrate was obtained in 2006. In a short-lived fish, Nothobranchius furzeri, with a median life span of nine weeks, a maximal dose of resveratrol increased the median lifespan by 56%. Compared to control fish at nine weeks, the fish supplemented with resveratrol showed significantly higher swimming activity and better learning to avoid an unpleasant stimulus. A slight increase of lifespan in young fish was caused by resveratrol, and it was hypothesized that its weak toxic action stimulated the defense mechanisms and resulted in the lifespan extension.[26]
Later the same year, Sinclair reported that resveratrol counteracted the detrimental effects of a high-fat diet in mice. The high-fat diet was compounded by adding hydrogenated coconut oil to the standard diet; it provided 60% of energy from fat, and the mice on it consumed about 30% more calories than the mice on standard diet and became obese and diabetic. Mice on the high-fat diet exhibited a high mortality rate compared to mice fed the standard diet; mice fed the high-fat diet plus 22 mg/kg resveratrol had a 30% lower risk of death than the mice on the high-fat diet alone, making their death rates similar to those on the standard diet. The supplement also partially corrected a subset of the abnormal gene expression profile and abnormal insulin and glucose metabolism. Resveratrol supplements did not change the levels of free fatty acids and cholesterol, however, which were much higher than in the mice on standard diet.[27]
Sinclair later reported that resveratrol treatment had a range of beneficial effects, but did not increase the longevity of nonobese, ad libitum–fed (freely-feeding) mice when started midlife.
Exercise and metabolism
Johan Auwerx in 2006 demonstrated that mice fed resveratrol have better treadmill endurance than controls. These results supported Sinclair's hypothesis that the effects of resveratrol are indeed due to the activation of the Sirtuin 1 gene.[28] The dose was 400 mg/kg of body weight (much higher than the 22 mg/kg of the Sinclair study). For an 80 kg (175 lb) person, the 400 mg/kg of body weight amount used in Auwerx's mouse study would total 30,000 mg/day. Compensating for the fact that humans have slower metabolic rates than mice would change the equivalent human dose to roughly 4000 mg/day.[29]
In a study of 123 Finnish adults, those born with certain increased variations of the SIRT1 gene had faster metabolisms, helping them to burn more energy, indicating the same pathway shown in the laboratory mice works in humans.[28] A later short-term study from Auwerx's laboratory in obese humans (similar to the obese mice in Auwerx's 2006 study) also found that resveratrol supplementation activated SIRT1 in this population.[30]
Cancer prevention
In 1997, Jang reported that topical resveratrol applications prevented skin cancer development in mice treated with a carcinogen.[31] There have since been many studies of the anti-cancer activity of resveratrol in animal models.[13] Resveratrol (1 mg/kg orally) reduced the number and size of the esophageal tumors in rats treated with a carcinogen;[32] and in several studies, small doses (0.02–8 mg/kg) of resveratrol, given prophylactically, reduced or prevented the development of intestinal and colon tumors in rats given different carcinogens.[33] Similarly, topical application of resveratrol in mice, both before and after the UVB exposure, inhibited the skin damage and decreased skin cancer incidence, however, oral resveratrol was ineffective in treating mice inoculated with melanoma cells. Resveratrol given orally also had no effect on leukemia and lung cancer;[33][34] however, injected intraperitoneally, 2.5 or 10 mg/kg of resveratrol slowed the growth of metastatic Lewis lung carcinomas in mice.[33][35]
Resveratrol treatment appeared to prevent the development of mammary tumors in animal models; however, it had no effect on the growth of existing tumors. Paradoxically, treatment of prepubertal mice with high doses of resveratrol enhanced formation of tumors. Injected in high doses into mice, resveratrol slowed the growth of neuroblastomas.[33]
All of the aforementioned in vivo studies have been in animal models in which the cancer has been artificially induced by some experimental means. Three other studies have investigated the effect of resveratrol on the risk of cancer in normal mice living out a normal lifespan; all of them have found resveratrol supplementation has no significant effect on the burden of tumors, nor on the rate of cancer death.[7][8][36]
As of 2007, no results of human clinical trials for cancer had been reported.[33] Clinical trials to investigate the effects on colon cancer and melanoma (skin cancer) are currently recruiting patients.[37] The study of pharmacokinetics of resveratrol in humans concluded, however, that even high doses of resveratrol might be insufficient to achieve the resveratrol concentrations required for the systemic prevention of cancer.[38]
This is consistent with the results from the animal cancer models, which indicate the in vivo effectiveness of resveratrol is limited by its poor systemic bioavailability.[39][40] The strongest evidence of anticancer action of resveratrol exists for tumors it can contact directly, such as skin and gastrointestinal tract tumors. For other cancers, the evidence is uncertain, even if massive doses of resveratrol are used.[33]
Cardioprotective effects
Moderate drinking of red wine has long been known to reduce the risk of heart disease.[41] This is best known as "the French paradox".[42][43][44]
Studies suggest resveratrol in red wine may play an important role in this phenomenon.[45] It achieves the effects by the following functions: (1) inhibition of vascular cell adhesion molecule expression;[46][47] (2) inhibition of vascular smooth muscle cell proliferation;[48][49][50][51] (3) stimulation of endothelial nitric oxide synthase (eNOS) activity;[52][53][54] (4) inhibition of platelet aggregation;[55][56][57][58] and (5) inhibition of LDL peroxidation.[59][60]
The cardioprotective effects of resveratrol also are theorized to be a form of preconditioning—the best method of cardioprotection, rather than direct therapy.[61] Study into the cardioprotective effects of resveratrol is based on the research of Dipak K. Das. However, he has been found guilty of scientific fraud, and many of his publications related to resveratrol have been retracted.[62][63] A 2011 study concludes, "Our data demonstrate that both melatonin and resveratrol, as found in red wine, protect the heart in an experimental model of myocardial infarction via the SAFE pathway."[64]
Antidiabetic effects
Studies have shown resveratrol possesses hypoglycemic and hypolipidemic effects in both streptozotocin (STZ)-induced diabetes rats and STZ-nicotinamide-induced diabetes rats. Resveratrol ameliorates common diabetes symptoms, such as polyphagia, polydipsia, and body weight loss.[65] Other diabetic animal model studies by different researchers have also demonstrated the antidiabetic effects of resveratrol.[27][28][66][67][68][69][70]
A press release from Sirtris Pharmaceuticals claimed that resveratrol lowered blood sugar levels in both Phase Ib and Phase IIa clinical trials.[71][72] This 28-day Phase 1b study was conducted privately in India with Sirtris as the sponsor, and was announced at an investor conference in 2008.[73] Although it has been alluded to in review articles (e.g.[74]), the study has never been published in a peer-reviewed scientific publication.
Subsequently, it was reported that high-dose resveratrol did improve insulin sensitivity and postmeal plasma glucose in older, overweight and obese subjects with impaired glucose tolerance.[75] On the other hand, resveratrol had no effects on plasma lipids, inflammatory markers, insulin sensitivity, or plasma glucose.[76]
Skin protection
The oxidative stress induced by ultraviolet radiation is one of the main causes for premature skin ageing. The photoprotective effects of several polyphenols known for their antioxidant properties, including resveratrol, have been investigated in silico and in topical application conditions.[77][78]
In cell culture experiment, pretreatment of UVB-induced keratinocytes with resveratrol inhibited activation of NF-κB pathway and increased cell survival, with a parallel reduction of reactive oxygen species.[79][80]
In several experiments on hairless mice, topical application of resveratrol significantly inhibited UVB-induced effects including skin hyperplasia, hydrogen peroxide generation, leucocytes infiltration and phosphorylation of survivin.[81][82][83]
A recent study[84] conducted on human keratinocyte cell cultures also demonstrated the photoprotective effects of resveratrol against UVA radiation. The results suggest that resveratrol acts on the Keap1-Nrf2 signaling pathway, wich is the major regulator of cytoprotective responses to oxidative stress. Resveratrol can degrade Keap1 protein, increase the Nrf2 level and facilitate Nrf2 accumulation in the nucleus, thereby protecting HaCaT cells from UVA-induced oxidative stress.
In 2013, a study designed to investigate the effects of resveratrate, a stable derivative of resveratrol, in human skin irradiated with acute UVA-UVB combination[85] reported several photoprotective effects. Resveratrate significantly attenuated tanning and erythema developments, sunburn damages and sunburn cell formation (i.e. apoptotic keratinocytes) in human skin in vivo. Resveratrate also inhibited the increase of the menalin content in the epidermis. This result was equivalent to an anti-oxydant formulation but resveratrate may inhibit the suntan at multiple points, whereas anti-oxydants may not.
Premature skin ageing from UV radiation results in damages to dermal connective tissues, which mainly contains collagen, thereby causing wrinkle formation. Another recent study.[86] demonstrated that resveratrol and metformin significantly inhibited the expression of matrix metallopeptidase 9 and protected collagen from degradation after UV radiation, through the activation of SirT1.
3H-resveratrol binding sites have been found in human epidermis. In human keratinocyte cell cultures, their activation reduced cell death caused by exposure to nitric oxyde free radical donor sodium nitroprusside (SNP) and by the release of nitric oxyde.[87] The authors concluded that the protective action of resveratrol may likely to be due to an anti-apoptotic effect, since at the same concentration level, resveratrol reduced the number of apoptotic cells as well as apoptotic events launched by SNP.
More and more companies are developing and selling products containing resveratrol, for nutritional or topic use, to protect and help skin fight against the signs of ageing skin. Human clinical trials, when available, seems to confirm the anti-ageing effects of resveratrol in skin. In a trial, patients using Caudalie products containing resveratrol experienced a 24% reduction in deep wrinkles reduction after 28 days.[88]
Other applications
- Neuroprotective effects
In isolated cell culture systems, resveratrol treatment reduces accumulation of beta-amyloid, a main culprit in Alzheimer's disease.[89] Dietary supplementation with resveratrol also significantly reduced plaque formation in the brains of animals with mutations that cause them to produce large amounts of beta-amyloid.[90] Researchers speculate that one possible mechanism is the ability of resveratrol to chelate (bind) copper.[90] Other studies have proposed that the inhibitor effect of resveratrol on amyloid plaque formation is mediated by the activation of AMP-activated protein kinase.[91] The neuroprotective effects have been confirmed in several animal model studies.[92][93][94][95][96] These effects may be in part caused by its effects as a reversible inhibitor of monoamine oxidase A (RIMA). A large, multicenter clinical trial of resveratrol versus placebo for adults diagnosed with probable Alzheimer's disease is currently underway, led by researchers at Georgetown University Medical Center.[97]
- Anti-inflammatory effects
The anti-inflammatory effects of resveratrol have been demonstrated in several animal model studies. In a rat model of carrageenan-induced paw edema, resveratrol inhibited both acute and chronic phases of the inflammatory process.[98] Similarly, preincubation with resveratrol decreased arachidonic acid release and COX-2 induction in mouse peritoneal macrophages stimulated with tumor promoter PMA, ROI, or lipopolysaccharides (LPS).[99] In an experimental rabbit inflammatory arthritis model, resveratrol showed promise as a potential therapy for arthritis. When administered to rabbits with induced inflammatory arthritis, resveratrol protected cartilage against the progression of inflammatory arthritis.[100]
- Antiviral effects
Resveratrol inhibits herpes simplex virus (HSV) types 1 and 2 replication by inhibition of an early step in the virus replication cycle. In vivo studies in mice found resveratrol inhibits or reduces HSV replication in the vagina and limits extravaginal disease. The skin of resveratrol-treated animals showed no apparent dermal toxicity, such as erythema, scaling, crusting, lichenification, or excoriation.[101][102][103] Studies also show resveratrol inhibits varicella-zoster virus, certain influenza viruses, respiratory viruses, and human cytomegalovirus. Furthermore, resveratrol synergistically enhances the anti-HIV-1 activity of several anti-HIV drugs.[104][105][106][107][108][109]
- Effect on testosterone levels
Trans-resveratrol supplementation increased testosterone levels in mice in vivo,[110] which has led to its marketing as a bodybuilding supplement. The antioxidant resveratrol also increases sperm production in rats.[111]
- Selective and reversible MAO-A inhibiting effects (RIMA)
Resveratrol has been found to be a potent and highly selective reversible inhibitor of monoamine oxidase type A (MAO-A) (RIMA) with an IC50 of 2.0μM and a Ki value of 2.5μM in rat brains. This effect is expected to be related to its potent antioxidant activity.[112]
- Opioid tolerance reduction
Injection of resveratrol into the spinal column was found to alleviate tolerance to opioids in rats subjected to long-term opioid exposure via a catheter. The effect seems to involve a reversal of the tolerance-associated increase in expression of receptors for the neurotransmitter N-Methyl-D-aspartic acid (NMDA), and blockage of a tolerance-associated increase of inflammation-promoting signaling substances, called cytokines.[113]
Sirtuin activation
There is an explicit link between resveratrol and sirtuins; specifically that SIRT1 could be directly activated through an allosteric mechanism common to chemically diverse STACs, including resveratrol—in other words, that an anti-aging protein in humans could be activated by resveratrol, at least in vitro and under certain experimental conditions.[114]
Pharmacokinetics
One way of administering resveratrol in humans may be buccal delivery, that is without swallowing, by direct absorption through tissues on the inside of the mouth. When one milligram of resveratrol in 50 ml 50% alcohol/ water solution was retained in the mouth for one minute before swallowing, 37 ng/ml of free resveratrol were measured in plasma two minutes later. This level of unchanged resveratrol in blood can only be achieved with 250 mg of resveratrol taken in a pill form.[115] However, the viability of a buccal delivery method is called into question due to the low aqueous solubility of the molecule. For a drug to be absorbed transmucosally it must be in free-form or dissolved.[116][117] Resveratrol fits the criteria for oral transmucosal dosing, except for this caveat. The low aqueous solubility greatly limits the amount that can be absorbed through the buccal mucosa, which is why the method has not been explored further. All resveratrol that is attempted to be taken buccally will fail to pass through the mucous membrane of the mouth and be absorbed as an oral dose,[118] however, a need to explore buccal delivery in future pharmaceutical formulations has been expressed.[117][119]
While 70% of orally administered resveratrol is absorbed its oral bioavailability is approximately 1% due to extensive hepatic gluconuridation and sulfation.[120] Only trace amounts (below 5 ng/ml) of unchanged resveratrol could be detected in the blood after 25 mg oral dose.[120] Even when a very large dose (2.5 and 5 g) was given as an uncoated pill, the concentration of resveratrol in blood failed to reach the level claimed to be necessary for the systemic cancer prevention.[38] A formulation of resveratrol in a chewing gum form is now in production, and this would be expected to achieve much higher blood levels than oral formulations. Resveratrol given in a proprietary formulation SRT-501 (3 or 5 g), developed by Sirtris Pharmaceuticals, reached five to eight times higher blood levels. These levels did approach the concentration necessary to exert the effects shown in animal models and in vitro experiments.[74] On May 5, 2010, however, GlaxoSmithKline (GSK) said it had suspended a small clinical trial of SRT501, a proprietary form of resveratrol, due to safety concerns, and terminated the study on December 2, 2010.[121] Sirtris Pharmaceuticals, which U.K.-based GlaxoSmithKline bought for $720 million in 2008, was developing the drug. GlaxoSmithKline is now focusing its efforts on more potent and selective SIRT1 activators—SRT2104 and SRT2379—both of which are involved in several exploratory clinical trials.[citation needed]
Full formal pharmacokinetics of oral resveratrol 2000 mg twice daily in humans, studying interaction with concurrent ethanol, quercetin, and fat meal has been published.[122] Mean peak serum resveratrol concentration was 1274 ng/ml at steady-state, which was reduced 46% by a fat meal at dosing. There was no effect of concurrent oral quercetin or ethanol. Healthy volunteers had frequently reported minor diarrhea, and laboratory measures identified slight changes in liver function tests and in serum potassium. No adverse effect on renal function was identified, although only eight healthy adults were observed in the two-week study.
In humans and rats less than 5% of the oral dose was observed as free resveratrol in blood plasma.[38][40][120][123][124] The most abundant resveratrol metabolites in humans, rats, and mice are trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate.[125] Walle suggests sulfate conjugates are the primary source of activity,[120] Wang et al. suggests the glucuronides,[126] and Boocock et al. also emphasized the need for further study of the effects of the metabolites, including the possibility of deconjugation to free resveratrol inside cells. The hypothesis that resveratrol from wine could have higher bioavailability than resveratrol from a pill [13][127] has been refuted by experimental data.[128][129] For example, after five men took 600 ml of red wine with the resveratrol content of 3.2 mg/l (total dose about 2 mg) before breakfast, unchanged resveratrol was detected in the blood of only two of them, and only in trace amounts (below 2.5 ng/ml). Resveratrol levels appeared to be slightly higher if red wine (600 ml of red wine containing 0.6 mg/ml resveratrol; total dose about 0.5 mg) was taken with a meal: trace amounts (1–6 ng/ml) were found in four out of ten subjects.[129] In another study, the pharmacokinetics of resveratrol (25 mg) did not change whether it was taken with vegetable juice, white wine, or white grape juice. The highest level of unchanged resveratrol in the serum (7–9 ng/ml) was achieved after 30 minutes, and it completely disappeared from blood after four hours.[128] The authors of both studies concluded the trace amounts of resveratrol reached in the blood are insufficient to explain the French paradox. The beneficial effects of wine apparently could be explained by the effects of alcohol [128] or the whole complex of substances wine contains;[129] for example, the cardiovascular benefits of wine appear to correlate with the content of procyanidins.[130]
Adverse effects
Long-term effects of using resveratrol are currently unknown.[11] One study has theorized it may stimulate the growth of human breast cancer cells, possibly because of resveratrol's chemical structure, which is similar to a phytoestrogen.[12][131] Other studies have found resveratrol intake is inversely associated with breast cancer risk, however, and acts to slow the progression of breast cancer that has been transplanted into mice.[132][133] Some studies suggest resveratrol slows the development of blood vessels, which suppresses tumors, but also slows healing.[134] Citing the evidence that resveratrol is estrogen antagonistic, some retailers of resveratrol advise that the compound may interfere with oral contraceptives and that women who are pregnant or intending to become pregnant should not use the product, while others advise that resveratrol should not be taken by children or young adults under eighteen, as no studies have shown how it affects their natural development. A small study found a single dose of up to 5 g of trans-resveratrol caused no serious adverse effects in healthy volunteers.[38]
Potential carcinogenicity
Resveratrol in common with other polyphenols, was found to be a strong topoisomerase inhibitor, sharing similarities to chemotherapeutic anticancer drugs, such as etoposide and doxorubicin.[135][136] This may simultaneously contribute to both the potential anticarcinogenic and carcinogenic properties of the substance in given circumstances. Harmful properties of resveratrol may be pronounced in the human fetus, as it has diminished detoxification systems. Therefore, resveratrol as commonly sold combined with other "bioflavonoids", should not be used by pregnant women.[137]
Mechanisms of action
The mechanisms of resveratrol's apparent effects on life extension are not fully understood, but they appear to mimic several of the biochemical effects of calorie restriction. Some studies indicates resveratrol activates Sirtuin 1[138] and PGC-1α and improves the functioning of the mitochondria.[28]
In cells treated with resveratrol, a fourteen-fold increase in the action of MnSOD (SOD2) is observed.[139] MnSOD reduces superoxide to hydrogen peroxide (H2O2), but H2O2 is not increased due to other cellular activity. Superoxide O2− is a byproduct of respiration in complexes 1 and 3 of the electron transport chain. It is "not highly toxic, [but] can extract an electron from biological membrane and other cell components, causing free radical chain reactions. Therefore it is essential for the cell to keep superoxide anions in check."[140] MnSOD reduces superoxide and thereby, confers resistance to mitochondrial dysfunction, permeability transition, and apoptotic death in various diseases.[141] It has been implicated in lifespan extension, inhibits cancer, (e.g. pancreatic cancer) [142][143] and provides resistance to reperfusion injury and irradiation damage.[144][145][146] These effects have also been observed with resveratrol. Robb et al. propose MnSOD is increased by the pathway RESV → SIRT1 / NAD+ → FOXO3a → MnSOD. Resveratrol has been shown to cause SIRT1 to cause migration of FOXO transcription factors to the nucleus,[147] which stimulates FOXO3a transcriptional activity [148] and it has been shown to enhance the sirtuin-catalyzed deacetylation (activity) of FOXO3a. MnSOD is known to be a target of FOXO3a, and MnSOD expression is strongly induced in cells overexpressing FOXO3a.[149] It has been reported too that the disproportional up-regulation of superoxide dismutase (SOD), catalse (CAT) and glutathion peroxidase (GPX) expression (high expression of MnSOD, but mild change in CAT or GPX) and their enzymatic activity in cancer cells results in the mitochondrial accumulation of H2O2, which in turn induces cancer cell apoptosis.[150]
Resveratrol interferes with all three stages of carcinogenesis—initiation, promotion and progression. Experiments in cell cultures of varied types and isolated subcellular systems in vitro imply many mechanisms in the pharmacological activity of resveratrol. These mechanisms include modulation of the transcription factor NF-κB,[151] inhibition of the cytochrome P450 isoenzyme CYP1A1[152] (although this may not be relevant to the CYP1A1-mediated bioactivation of the procarcinogen benzo(a)pyrene),[153] alterations in androgenic [154] actions, and expression and activity of cyclooxygenase (COX) enzymes. In vitro, resveratrol "inhibited the proliferation of human pancreatic cancer cell lines." In some lineages of cancer cell culture, resveratrol has been shown to induce apoptosis, which means it kills cells and may kill cancer cells.[154][155][156][157][158][159] Resveratrol has been shown to induce Fas/Fas ligand mediated apoptosis, p53 and cyclins A, B1, and cyclin-dependent kinases cdk 1 and 2. Resveratrol also possesses antioxidant and anti-angiogenic properties.[134][160][161]
Resveratrol was reported to be effective against neuronal cell dysfunction and cell death, and, in theory, could be effective against diseases such as Huntington's disease and Alzheimer's disease.[89][162] Again, this has not yet been tested in humans for any disease.
Resveratrol has direct inhibitory action on cardiac fibroblasts, and may inhibit the progression of cardiac fibrosis.[163]
Resveratrol also significantly increases natural testosterone production from being both a selective estrogen receptor modulator [111][164] and an aromatase inhibitor.[165]
Resveratrol increased intracellular glutathione levels via Nrf2-dependent upregulation of gamma-glutamylcysteine ligase in lung epithelial cells, which protected them against cigarette smoke extract-induced oxidative stress.[166]
Another potentially important mechanism common to both resveratrol supplementation and caloric restriction is the modulation of autophagy.[167] SIRT1 is a hypothesized target of both resveratrol and caloric restriction, and has been shown to facilitate autophagy through the inhibition of mTOR, which itself negatively regulates autophagy.[168]
In 2012, it was shown that resveratrol is capable of competitively inhibiting various phosphodiesterases, which results in an increase in cytosolic concentration of cAMP, which acts as a second messenger for the activation of the pathway Epac1/CaMKKβ/AMPK/SIRT1/PGC-1α. This rise of cAMP concentration allows an increase in oxidation of fatty acids, mitochondrial biogenesis, mitochondrial respiration, and gluconeogenesis.[169][170]
Resveratrol is one of the compounds that may prevent negative effects of advanced glycation end-products (AGEs), at least in vitro.[171]
Chemical and physical properties
Resveratrol (3,5,4'-trihydroxystilbene) is a stilbenoid, a derivate of stilbene.
It exists as two geometric isomers: cis- (Z) and trans- (E), with the trans-isomer shown in the top image. The trans- and cis-resveratrol can be either free or bound to glucose.[172]
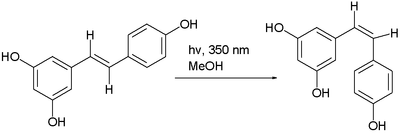
The trans- form can undergo isomerisation to the cis- form when exposed to ultraviolet irradiation,[173] a process called photoisomerization:[174]
Recently, it is noted that ultraviolet irradiation to cis-resveratrol induces further photochemical reaction, produces a fluorescent molecule named "Resveratrone".[175]
Trans-resveratrol in the powder form was found to be stable under "accelerated stability" conditions of 75% humidity and 40 °C in the presence of air.[176] Resveratrol content also was stable in the skins of grapes and pomace taken after fermentation and stored for a long period.[177] lH- and 13C-NMR data for the four most common forms of resveratrols are reported in literature.[172]
Metabolism
Resveratrol gets extensively metabolized in the body. Liver and gut are the major site of its metabolism. Lungs are also involved in its metabolism, with inter-species difference in its pulmonary metabolism.[178]
Biosynthesis
Resveratrol is produced in plants with the help of the enzyme, resveratrol synthase.[179]
Biotransformation
The grapevine fungal pathogen Botrytis cinerea is able to oxidise resveratrol into metabolites showing attenuated antifungal activities. Those include the resveratrol dimers restrytisol A, B, and C, resveratrol trans-dehydrodimer, leachinol F, and pallidol.[180] The soil bacterium Bacillus cereus can be used to transform resveratrol into piceid (resveratrol 3-O-beta-D-glucoside).[181]
Occurrences
In plants
Resveratrol was originally isolated by Takaoka from the roots of hellebore in 1940, and later, in 1963, from the roots of Japanese knotweed. It attracted wider attention only in 1992, however, when its presence in wine was suggested as the explanation for cardioprotective effects of wine.[13]
In grapes, trans-resveratrol is a phytoalexin produced against the growth of fungal pathogens such as Botrytis cinerea.[182] Its presence in Vitis vinifera grapes can also be constitutive, with accumulation in ripe berries of different levels of bound and free resveratrols, according to the genotype.[183] In grapes, resveratrol is found primarily in the skin,[184] and, in muscadine grapes, also in the seeds.[185] The amount found in grape skins also varies with the grape cultivar, its geographic origin, and exposure to fungal infection. The amount of fermentation time a wine spends in contact with grape skins is an important determinant of its resveratrol content.[172][184]
It is also found in Pinus strobus, the eastern white pine.
In foods
The levels of resveratrol found in food varies greatly. Red wine contains between 0.2 and 5.8 mg/l,[186] depending on the grape variety, while white wine has much less, because red wine is fermented with the skins, allowing the wine to extract the resveratrol, whereas white wine is fermented after the skin has been removed.[184][187] The composition of wine is different from that of grapes since the extraction of resveratrols from grapes depends on the duration of the skin contact, and the resveratrol 3-glucosides are in part hydrolised, yielding both trans- and cis-resveratrol.[172] A number of reports have indicated muscadine grapes may contain high concentrations of resveratrol, and that wines produced from these grapes, both red and white, may contain more than 40 mg/l,[185][188] however, subsequent studies have found little or no resveratrol in different varieties of muscadine grapes.[189][190]
One of the most promising sources is peanuts, especially sprouted peanuts where the content rivals that in grapes. Before sprouting, it was in the range of 2.3 to 4.5 μg/g, and after sprouting, in the range of 11.7 to 25.7 μg/g depending upon peanut cultivar.[191]
The fruit of the mulberry (esp. the skin)[192] is a source, and is sold as a nutritional supplement.
Cocoa powder, baking chocolate, and dark chocolate also have low levels of resveratrol in normal consumption quantities (0.35 to 1.85 mg/kg).[193]
Content in wines and grape juice
| Beverage | Total resveratrol (mg/l)[184][185] | Total resveratrol (mg/150ml)[184][185] |
|---|---|---|
| Red wine (global) | 1.98 – 7.13 | 0.30 – 1.07 |
| Red wine (Spanish) | 1.92 – 12.59 | 0.29 – 1.89 |
| Red grape juice (Spanish) | 1.14 – 8.69 | 0.17 – 1.30 |
| Rose wine (Spanish) | 0.43 – 3.52 | 0.06 – 0.53 |
| Pinot noir | 0.40 – 2.0 | 0.06 – 0.30 |
| White wine (Spanish) | 0.05 – 1.80 | 0.01 – 0.27 |
The trans-resveratrol concentration in 40 Tuscan wines ranged from 0.3 to 2.1 mg/l in the 32 red wines tested and had a maximum of 0.1 mg/l in the 8 white wines in the test. Both the cis- and trans-isomers of resveratrol were detected in all tested samples. cis-resveratrol levels were comparable to those of the trans-isomer. They ranged from 0.5 mg/l to 1.9 mg/l in red wines and had a maximum of 0.2 mg/l in white wines.[194]
In a review of published resveratrol concentrations, the average in red wines is 1.9 ± 1.7 mg trans-resveratrol/L (8.2 ± 7.5 μM), ranging from nondetectable levels to 14.3 mg/l (62.7 μM) trans-resveratrol. Levels of cis-resveratrol follow the same trend as trans-resveratrol.[195]
Reports suggest some aspect of the wine making process converts piceid to resveratrol in wine, as wine seems to have twice the average resveratrol concentration of the equivalent commercial juices.[185]
In general, wines made from grapes of the Pinot Noir and St. Laurent varieties showed the highest level of trans-resveratrol, though no wine or region can yet be said to produce wines with significantly higher concentrations than any other wine or region.[195]
Content in selected foods
| Food | Serving | Total resveratrol (mg)[193][196] |
|---|---|---|
| Peanuts (raw) | 1 c (146 g) | 0.01 – 0.26 |
| Peanuts (boiled) | 1 c (180 g) | 0.32 – 1.28 |
| Peanut butter | 1 c (258 g) | 0.04 – 0.13 |
| Red grapes | 1 c (160 g) | 0.24 – 1.25 |
| Cocoa powder | 1 c (200 g) | 0.28 – 0.46 |
Ounce for ounce, peanuts have about half as much resveratrol as red wine. The average amount in peanuts in the marketplace is 79.4 µg/ounce.
In comparison, some red wines contain approximately 160 µg/fluid ounce.[197] Resveratrol was detected in grape, cranberry, and wine samples. Concentrations ranged from 1.56 to 1042 nmol/g in Concord grape products, and from 8.63 to 24.84 µmol/L in Italian red wine. The concentrations of resveratrol were similar in cranberry and grape juice at 1.07 and 1.56 nmol/g, respectively.[198]
Blueberries have about twice as much resveratrol as bilberries, but there is great regional variation. These fruits have less than 10% of the resveratrol of grapes. Cooking or heat processing of these berries will contribute to the degradation of resveratrol, reducing it by up to half.[199]
Supplementation
As a result of extensive news coverage,[200][201] sales of supplements greatly increased in 2006.[202] This was despite the existence of studies cautioning that benefits to humans are unproven.[202][203][204]
Supplements vary in purity and can contain anywhere from 50 percent to 99 percent resveratrol. Many brands consist of an unpurified extract of Japanese knotweed (Polygonum cuspidatum), an introduced species in many countries. These contain about 50 percent resveratrol by weight, as well as emodin, which, while considered safe in moderate quantities, can have a laxative effect in high amounts.[205] Resveratrol can be produced from its glucoside piceid from Japanese knotweed fermented by Aspergillus oryzae.[16]
Harvard University scientist and professor David Sinclair is often quoted in online ads for resveratrol supplements, many of which imply endorsement of the advertized product; however, Sinclair, who has studied resveratrol extensively, has gone on record in Bloomberg Businessweek to say he never uttered many of the statements attributed to him on these sites.[206]
Related compounds
- Dihydro-resveratrol
- Epsilon-viniferin and Pallidol, two different resveratrol dimers
- Trans-diptoindonesin B, a resveratrol trimer
- Hopeaphenol, a resveratrol tetramer
- Oxyresveratrol, the aglycone of mulberroside A, a compound found in Morus alba, the white mulberry[207]
- Piceatannol, an active metabolite of resveratrol found in red wine
- Piceid, a resveratrol glucoside
- Pterostilbene, a doubly methylated resveratrol
- 4'-Methoxy-(E)-resveratrol 3-O-rutinoside, a compound found in the stem bark of Boswellia dalzielii[208]
See also
References
- ^ a b Camont L, Cottart CH, Rhayem Y, Nivet-Antoine V, Djelidi R, Collin F, Beaudeux JL, Bonnefont-Rousselot D (February 2009). "Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions". Anal. Chim. Acta. 634 (1): 121–8. doi:10.1016/j.aca.2008.12.003. PMID 19154820.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Resveratrol MSDS on Fisher Scientific website
- ^ Bechmann LP, Zahn D, Gieseler RK, Fingas CD, Marquitan G, Jochum C, Gerken G, Friedman SL, Canbay A (June 2009). "Resveratrol amplifies profibrogenic effects of free fatty acids on human hepatic stellate cells". Hepatol. Res. 39 (6): 601–8. doi:10.1111/j.1872-034X.2008.00485.x. PMC 2893585. PMID 19207580.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Resveratrol MSDS on www.sigmaaldrich.com
- ^ "Pharma seeks genetic clues to healthy aging". Reuters. 6 April 2010.
- ^ http://www.sciencedaily.com/releases/2013/12/131219130738.htm
- ^ a b Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R (August 2008). "Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span". Cell Metab. 8 (2): 157–68. doi:10.1016/j.cmet.2008.06.011. PMC 2538685. PMID 18599363.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (February 2011). "Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice". J. Gerontol. A Biol. Sci. Med. Sci. 66 (2): 191–201. doi:10.1093/gerona/glq178. PMC 3021372. PMID 20974732.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE (January 2013). "Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice". J. Gerontol. A Biol. Sci. Med. Sci. 68 (1): 6–16. doi:10.1093/gerona/gls070. PMC 3598361. PMID 22451473.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Micronutrient Information Center: Resveratrol". Linus Pauling Institute at Oregon State University. Retrieved 2012-01-13.
- ^ a b Healy, Melissa (August 31, 2009). "Selling resveratrol: Wonder drug or snake oil?". The Connecticut Post. The Los Angeles Times.
- ^ a b Gehm BD, McAndrews JM, Chien PY, Jameson JL (December 1997). "Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor". Proc. Natl. Acad. Sci. U.S.A. 94 (25): 14138–43. doi:10.1073/pnas.94.25.14138. PMC 28446. PMID 9391166.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Baur JA, Sinclair DA (June 2006). "Therapeutic potential of resveratrol: the in vivo evidence". Nature Reviews Drug Discovery. 5 (6): 493–506. doi:10.1038/nrd2060. PMID 16732220.
- ^ Farina A, Ferranti C, Marra C (March 2006). "An improved synthesis of resveratrol". Nat. Prod. Res. 20 (3): 247–52. doi:10.1080/14786410500059532. PMID 16401555.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Trantas E, Panopoulos N, Ververidis F (November 2009). "Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae". Metab. Eng. 11 (6): 355–66. doi:10.1016/j.ymben.2009.07.004. PMID 19631278.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wang H, Liu L, Guo YX, Dong YS, Zhang DJ, Xiu ZL (June 2007). "Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae". Appl. Microbiol. Biotechnol. 75 (4): 763–8. doi:10.1007/s00253-007-0874-3. PMID 17333175.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yao CS, Lin M, Liu X, Wang YH (April 2005). "Stilbene derivatives from Gnetum cleistostachyum". J Asian Nat Prod Res. 7 (2): 131–7. doi:10.1080/10286020310001625102. PMID 15621615.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Resveratrol, a new phenolic compound, from Veratrum grandiflorum. M Takaoka, Journal of the Chemical Society of Japan, 1939, volume 60, pages 1090-1100 (abstract)
- ^ Schröder, Joachim (March 6, 2010). "Discovery of resveratrol". Resveratrol.[self-published source?]
- ^ Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (September 2003). "Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan". Nature. 425 (6954): 191–6. doi:10.1038/nature01960. PMID 12939617.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D (August 2004). "Sirtuin activators mimic caloric restriction and delay ageing in metazoans". Nature. 430 (7000): 686–9. doi:10.1038/nature02789. PMID 15254550.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gruber J, Tang SY, Halliwell B (April 2007). "Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans". Annals of the New York Academy of Sciences. 1100: 530–42. doi:10.1196/annals.1395.059. PMID 17460219.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cite error: The named reference
Bass2007was invoked but never defined (see the help page). - ^ Cite error: The named reference
Kaeberlein2005was invoked but never defined (see the help page). - ^ Cite error: The named reference
Zou2009was invoked but never defined (see the help page). - ^ Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A (February 2006). "Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate". Curr. Biol. 16 (3): 296–300. doi:10.1016/j.cub.2005.12.038. PMID 16461283.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (November 2006). "Resveratrol improves health and survival of mice on a high-calorie diet". Nature. 444 (7117): 337–42. doi:10.1038/nature05354. PMID 17086191.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J (December 2006). "Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha". Cell. 127 (6): 1109–22. doi:10.1016/j.cell.2006.11.013. PMID 17112576.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wade N (November 16, 2006). "Red Wine Ingredient Increases Endurance, Study Shows". New York Times.
- ^ Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P (November 2011). "Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans". Cell Metab. 14 (5): 612–22. doi:10.1016/j.cmet.2011.10.002. PMID 22055504.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (January 1997). "Cancer chemopreventive activity of resveratrol, a natural product derived from grapes". Science. 275 (5297): 218–20. doi:10.1126/science.275.5297.218. PMID 8985016.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M (September 2002). "Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol". Carcinogenesis. 23 (9): 1531–6. doi:10.1093/carcin/23.9.1531. PMID 12189197.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL (November 2007). "Resveratrol: a review of preclinical studies for human cancer prevention". Toxicol. Appl. Pharmacol. 224 (3): 274–83. doi:10.1016/j.taap.2006.12.025. PMC 2083123. PMID 17306316.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC (July 2002). "Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes". J. Nutr. 132 (7): 2076–81. PMID 12097696.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kimura Y, Okuda H (June 2001). "Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice". J. Nutr. 131 (6): 1844–9. PMID 11385077.
- ^ Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA (2008). Tomé, Daniel (ed.). "A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice". PLoS ONE. 3 (6): e2264. doi:10.1371/journal.pone.0002264. PMC 2386967. PMID 18523577.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Resveratrol. From Clinicaltrials.gov. Retrieved August 15, 2008.[non-primary source needed]
- ^ a b c d Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE (June 2007). "Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent". Cancer Epidemiol. Biomarkers Prev. 16 (6): 1246–52. doi:10.1158/1055-9965.EPI-07-0022. PMID 17548692.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Niles RM, Cook CP, Meadows GG, Fu YM, McLaughlin JL, Rankin GO (October 2006). "Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth". J. Nutr. 136 (10): 2542–6. PMC 1612582. PMID 16988123.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wenzel E, Soldo T, Erbersdobler H, Somoza V (May 2005). "Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats". Mol Nutr Food Res. 49 (5): 482–94. doi:10.1002/mnfr.200500003. PMID 15779067.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Szmitko PE, Verma S (January 2005). "Cardiology patient pages. Red wine and your heart". Circulation. 111 (2): e10–1. doi:10.1161/01.CIR.0000151608.29217.62. PMID 15657377.
- ^ Ferrières J (January 2004). "The French paradox: lessons for other countries". Heart. 90 (1): 107–11. doi:10.1136/heart.90.1.107. PMC 1768013. PMID 14676260.
- ^ Simini B (January 2000). "Serge Renaud: from French paradox to Cretan miracle". Lancet. 355 (9197): 48. doi:10.1016/S0140-6736(05)71990-5. PMID 10615898.
- ^ Renaud S, de Lorgeril M (June 1992). "Wine, alcohol, platelets, and the French paradox for coronary heart disease". Lancet. 339 (8808): 1523–6. doi:10.1016/0140-6736(92)91277-F. PMID 1351198.
- ^ Kopp P (June 1998). "Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'?". Eur. J. Endocrinol. 138 (6): 619–20. doi:10.1530/eje.0.1380619. PMID 9678525.
- ^ Ferrero ME, Bertelli AE, Fulgenzi A, Pellegatta F, Corsi MM, Bonfrate M, Ferrara F, De Caterina R, Giovannini L, Bertelli A (December 1998). "Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium". Am. J. Clin. Nutr. 68 (6): 1208–14. PMID 9846848.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rotondo S, Rajtar G, Manarini S, Celardo A, Rotillo D, de Gaetano G, Evangelista V, Cerletti C (April 1998). "Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function". Br. J. Pharmacol. 123 (8): 1691–9. doi:10.1038/sj.bjp.0701784. PMC 1565338. PMID 9605577.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Haider UG, Roos TU, Kontaridis MI, Neel BG, Sorescu D, Griendling KK, Vollmar AM, Dirsch VM (July 2005). "Resveratrol inhibits angiotensin II- and epidermal growth factor-mediated Akt activation: role of Gab1 and Shp2". Mol. Pharmacol. 68 (1): 41–8. doi:10.1124/mol.104.005421. PMID 15849355.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang Z, Chen Y, Labinskyy N, Hsieh TC, Ungvari Z, Wu JM (July 2006). "Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts". Biochem. Biophys. Res. Commun. 346 (1): 367–76. doi:10.1016/j.bbrc.2006.05.156. PMID 16759640.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Poussier B, Cordova AC, Becquemin JP, Sumpio BE (December 2005). "Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis". J. Vasc. Surg. 42 (6): 1190–7. doi:10.1016/j.jvs.2005.08.014. PMID 16376213.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kurin, Elena; Atanasov, Atanas; Donath, Oliver; Heiss, Elke; Dirsch, Verena; Nagy, Milan (2012). "Synergy Study of the Inhibitory Potential of Red Wine Polyphenols on Vascular Smooth Muscle Cell Proliferation". Planta Medica. 78 (8): 772–8. doi:10.1055/s-0031-1298440. PMID 22499559.
- ^ Duffy, Stephen J.; Vita, Joseph A. (2003). "Effects of phenolics on vascular endothelial function". Current Opinion in Lipidology. 14 (1): 21–7. doi:10.1097/01.mol.0000052857.26236.f2. PMID 12544657.
- ^ Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Förstermann U (September 2002). "Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase". Circulation. 106 (13): 1652–8. doi:10.1161/01.CIR.0000029925.18593.5C. PMID 12270858.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chen CK, Pace-Asciak CR (March 1996). "Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta". Gen. Pharmacol. 27 (2): 363–6. doi:10.1016/0306-3623(95)02001-2. PMID 8919657.
- ^ Olas B, Wachowicz B (August 2005). "Resveratrol, a phenolic antioxidant with effects on blood platelet functions". Platelets. 16 (5): 251–60. doi:10.1080/09537100400020591. PMID 16011975.
- ^ Stef G, Csiszar A, Lerea K, Ungvari Z, Veress G (August 2006). "Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance". J. Cardiovasc. Pharmacol. 48 (2): 1–5. doi:10.1097/01.fjc.0000238592.67191.ab. PMID 16954814.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang, Z; Zou, J; Huang, Y; Cao, K; Xu, Y; Wu, JM (2002). "Effect of resveratrol on platelet aggregation in vivo and in vitro". Chinese medical journal. 115 (3): 378–80. PMID 11940369.
- ^ Pace-Asciak, Cecil R.; Hahn, Susan; Diamandis, Eleftherios P.; Soleas, George; Goldberg, David M. (1995). "The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease". Clinica Chimica Acta. 235 (2): 207–19. doi:10.1016/0009-8981(95)06045-1. PMID 7554275.
- ^ Frémont L, Belguendouz L, Delpal S (1999). "Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids". Life Sci. 64 (26): 2511–21. doi:10.1016/S0024-3205(99)00209-X. PMID 10403511.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A (May 2007). "Resveratrol increases vascular oxidative stress resistance". Am. J. Physiol. Heart Circ. Physiol. 292 (5): H2417–24. doi:10.1152/ajpheart.01258.2006. PMID 17220179.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Das DK, Maulik N (February 2006). "Resveratrol in cardioprotection: a therapeutic promise of alternative medicine". Mol. Interv. 6 (1): 36–47. doi:10.1124/mi.6.1.7. PMID 16507749.
- ^ Weir, William; Megan, Kathleen (January 11, 2012). "Investigation Finds UConn Professor Fabricated Research - Work Focused On Resveratrol, Chemical In Red Wine". Hartford Courant.
- ^ Retraction Watch
- ^ Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S (May 2011). "Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection". J. Pineal Res. 50 (4): 374–80. doi:10.1111/j.1600-079X.2010.00853.x. PMID 21342247.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Su HC, Hung LM, Chen JK (June 2006). "Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats". Am. J. Physiol. Endocrinol. Metab. 290 (6): E1339–46. doi:10.1152/ajpendo.00487.2005. PMID 16434553.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM (July 2008). "Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways". Diabetes. 57 (7): 1814–23. doi:10.2337/db07-1750. PMC 2453636. PMID 18426865.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Palsamy P, Subramanian S (November 2008). "Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats". Biomed. Pharmacother. 62 (9): 598–605. doi:10.1016/j.biopha.2008.06.037. PMID 18675532.
- ^ Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K (2006). "Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats". Pharmacology. 76 (2): 69–75. doi:10.1159/000089720. PMID 16286809.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Corrêa M, da Rosa MM, Rubin MA, Chitolina Schetinger MR, Morsch VM (May 2009). "Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats". Eur. J. Pharmacol. 610 (1–3): 42–8. doi:10.1016/j.ejphar.2009.03.032. PMID 19303406.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik G, Menon VP, Bagchi D, Maulik N (December 2008). "Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium". J. Cell. Mol. Med. 12 (6A): 2350–61. doi:10.1111/j.1582-4934.2008.00251.x. PMID 18266981.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Sirtris Announces SRT501 Lowers Glucose in Twice-Daily Dosing Clinical Trial; Study Suggests Dose Response for Proprietary Formulation of Resveratrol in Type 2 Diabetics" (Press release). Sirtris Pharmaceuticals. April 17, 2008. Retrieved August 9, 2010.
- ^ "Sirtris Continues to Lead The Way In Resveratrol Based Research". My Resveratrol Experience. 2009-04-10. Retrieved 2011-01-22.
- ^ "Sirtris Announces Positive Results with Proprietary Version of Resveratrol, SRT501, in a Phase 1b Type 2 Diabetes Clinical Study" (Press release). Sirtris Pharmaceuticals. January 7, 2008. Retrieved November 2, 2013.
- ^ a b Elliott PJ, Jirousek M (April 2008). "Sirtuins: novel targets for metabolic disease". Current Opinion in Investigational Drugs. 9 (4): 371–8. PMID 18393104.
- ^ Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N (December 2012). "Pilot study of resveratrol in older adults with impaired glucose tolerance". J. Gerontol. A Biol. Sci. Med. Sci. 67 (12): 1307–12. doi:10.1093/gerona/glr235. PMC 3670158. PMID 22219517.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, Klein S (November 2012). "Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance". Cell Metab. 16 (5): 658–64. doi:10.1016/j.cmet.2012.09.015. PMC 3496026. PMID 23102619.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Afaq, Farrukh; Mukhtar, Hasan (2006). "Botanical antioxidants in the prevention of photocarcinogenesis and photoaging". Experimental Dermatology. 15 (9): 678–84. doi:10.1111/j.1600-0625.2006.00466.x. PMID 16881964.
- ^ Baliga, Manjeshwar S.; Katiyar, Santosh K. (2006). "Chemoprevention of photocarcinogenesis by selected dietary botanicals". Photochemical & Photobiological Sciences. 5 (2): 243–53. doi:10.1039/b505311k. PMID 16465310.
- ^ Adhami, VM; Afaq, F; Ahmad, N (2003). "Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol". Neoplasia. 5 (1): 74–82. PMC 1502124. PMID 12659672.
- ^ Park, K; Lee, JH (2008). "Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway". Oncology reports. 19 (2): 413–7. PMID 18202789.
- ^ Afaq, Farrukh; Adhami, Vaqar Mustafa; Ahmad, Nihal (2003). "Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice☆☆Part of this work was conducted at the Department of Dermatology, Case Western Reserve University and the Research Institute of University Hospitals of Cleveland, 11100 Euclid Avenue, Cleveland, Ohio 44106". Toxicology and Applied Pharmacology. 186 (1): 28–37. doi:10.1016/S0041-008X(02)00014-5. PMID 12583990.
- ^ Reagan-Shaw, Shannon; Afaq, Farrukh; Aziz, Moammir Hasan; Ahmad, Nihal (2004). "Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin". Oncogene. 23 (30): 5151–60. doi:10.1038/sj.onc.1207666. PMID 15122319.
- ^ Aziz, Moammir Hasan; Afaq, Farrukh; Ahmad, Nihal (2007). "Prevention of Ultraviolet-B Radiation Damage by Resveratrol in Mouse Skin is Mediated via Modulation in Survivin¶". Photochemistry and Photobiology. 81 (1): 25–31. doi:10.1111/j.1751-1097.2005.tb01518.x. PMID 15469386.
- ^ Liu, Yong; Chan, Fangxiao; Sun, Haimei; Yan, Jihong; Fan, Dongying; Zhao, Dongzhi; An, Jing; Zhou, Deshan (2011). "Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression". European Journal of Pharmacology. 650 (1): 130–7. doi:10.1016/j.ejphar.2010.10.009. PMID 20951123.
- ^ Wu, Y.; Jia, L.-L.; Zheng, Y.-N.; Xu, X.-G.; Luo, Y.-J.; Wang, B.; Chen, J.Z.S.; Gao, X.-H.; Chen, H.-D.; Matsui, M.; Li, Y.-H. (2013). "Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation". Journal of the European Academy of Dermatology and Venereology. 27 (3): 345–50. doi:10.1111/j.1468-3083.2011.04414.x. PMID 22221158.
- ^ Lee, Ji-Seon; Park, Keung-Young; Min, Hyung-Geun; Lee, Seung Jae; Kim, Jin-Ju; Choi, Joon-Seok; Kim, Won-Serk; Cha, Hyuk-Jin (2010). "Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue". Experimental Dermatology. 19 (12): 1060–6. doi:10.1111/j.1600-0625.2010.01129.x. PMID 20812964.
- ^ Bastianetto, Stéphane; Dumont, Yvan; Duranton, Albert; Vercauteren, Freya; Breton, Lionel; Quirion, Rémi (2010). Deli, Maria A (ed.). "Protective Action of Resveratrol in Human Skin: Possible Involvement of Specific Receptor Binding Sites". PLoS ONE. 5 (9): e12935. doi:10.1371/journal.pone.0012935. PMC 2944869. PMID 20886076.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Das, Dipak K.; Vasanthi, Hannah (2013). "Resveratrol in Dermal Health". Bioactive Dietary Factors and Plant Extracts in Dermatology. pp. 177–87. doi:10.1007/978-1-62703-167-7_18. ISBN 978-1-62703-166-0.
- ^ a b Marambaud P, Zhao H, Davies P (November 2005). "Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides". J. Biol. Chem. 280 (45): 37377–82. doi:10.1074/jbc.M508246200. PMID 16162502.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE (February 2009). "Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease". Neurochem. Int. 54 (2): 111–8. doi:10.1016/j.neuint.2008.10.008. PMC 2892907. PMID 19041676.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P (March 2010). "AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism". J. Biol. Chem. 285 (12): 9100–1. doi:10.1074/jbc.M109.060061. PMC 2838330. PMID 20080969.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Anekonda TS (September 2006). "Resveratrol--a boon for treating Alzheimer's disease?". Brain Res Rev. 52 (2): 316–26. doi:10.1016/j.brainresrev.2006.04.004. PMID 16766037.
- ^ Sharma M, Gupta YK (October 2002). "Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats". Life Sci. 71 (21): 2489–98. doi:10.1016/S0024-3205(02)02083-0. PMID 12270754.
- ^ Kumar P, Padi SS, Naidu PS, Kumar A (September 2006). "Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms". Behav Pharmacol. 17 (5–6): 485–92. doi:10.1097/00008877-200609000-00014. PMID 16940769.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yang YB, Piao YJ (July 2003). "Effects of resveratrol on secondary damages after acute spinal cord injury in rats". Acta Pharmacol. Sin. 24 (7): 703–10. PMID 12852839.
- ^ Sinha K, Chaudhary G, Gupta YK (June 2002). "Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats". Life Sci. 71 (6): 655–65. doi:10.1016/S0024-3205(02)01691-0. PMID 12072154.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Clinical trial number NCT01504854 for "Resveratrol for Alzheimer's Disease" at ClinicalTrials.gov
- ^ Gentilli M, Mazoit JX, Bouaziz H, Fletcher D, Casper RF, Benhamou D, Savouret JF (February 2001). "Resveratrol decreases hyperalgesia induced by carrageenan in the rat hind paw". Life Sci. 68 (11): 1317–21. doi:10.1016/S0024-3205(00)01018-3. PMID 11233998.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tsai SH, Lin-Shiau SY, Lin JK (February 1999). "Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol". Br. J. Pharmacol. 126 (3): 673–80. doi:10.1038/sj.bjp.0702357. PMC 1565862. PMID 10188978.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B (April 2007). "Effects of resveratrol in inflammatory arthritis". Inflammation. 30 (1–2): 1–6. doi:10.1007/s10753-006-9012-0. PMID 17115116.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, Pokabla CM, DeLucia AL (October 1999). "Resveratrol inhibition of herpes simplex virus replication". Antiviral Res. 43 (3): 145–55. doi:10.1016/S0166-3542(99)00042-X. PMID 10551373.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Docherty JJ, Fu MM, Hah JM, Sweet TJ, Faith SA, Booth T (September 2005). "Effect of resveratrol on herpes simplex virus vaginal infection in the mouse". Antiviral Res. 67 (3): 155–62. doi:10.1016/j.antiviral.2005.06.008. PMID 16125258.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Docherty JJ, Smith JS, Fu MM, Stoner T, Booth T (January 2004). "Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice". Antiviral Res. 61 (1): 19–26. doi:10.1016/j.antiviral.2003.07.001. PMID 14670590.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Docherty JJ, Sweet TJ, Bailey E, Faith SA, Booth T (December 2006). "Resveratrol inhibition of varicella-zoster virus replication in vitro". Antiviral Res. 72 (3): 171–7. doi:10.1016/j.antiviral.2006.07.004. PMID 16899306.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Guan WD, Yang ZF, Liu N, Qin S, Zhang FX, Zhu YT (September 2008). "[In vitro experimental study on the effect of resveratrol against several kinds of respiroviruses]". Zhong Yao Cai (in Chinese). 31 (9): 1388–90. PMID 19180966.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, Ciriolo MR, Garaci E (May 2005). "Inhibition of influenza A virus replication by resveratrol". J. Infect. Dis. 191 (10): 1719–29. doi:10.1086/429694. PMID 15838800.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li YQ, Li ZL, Zhao WJ, Wen RX, Meng QW, Zeng Y (September 2006). "Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication". Eur J Med Chem. 41 (9): 1084–9. doi:10.1016/j.ejmech.2006.03.024. PMID 16875760.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Evers DL, Wang X, Huong SM, Huang DY, Huang ES (August 2004). "3,4',5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling". Antiviral Res. 63 (2): 85–95. doi:10.1016/j.antiviral.2004.03.002. PMID 15302137.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Heredia A, Davis C, Redfield R (November 2000). "Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol". J. Acquir. Immune Defic. Syndr. 25 (3): 246–55. doi:10.1097/00126334-200011010-00006. PMID 11115955.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shin S, Jeon JH, Park D, Jang MJ, Choi JH, Choi BH, Joo SS, Nahm SS, Kim JC, Kim YB (January 2008). "trans-Resveratrol relaxes the corpus cavernosum ex vivo and enhances testosterone levels and sperm quality in vivo". Arch. Pharm. Res. 31 (1): 83–7. doi:10.1007/s12272-008-1124-7. PMID 18277612.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Juan ME, González-Pons E, Munuera T, Ballester J, Rodríguez-Gil JE, Planas JM (April 2005). "trans-Resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats". J. Nutr. 135 (4): 757–60. PMID 15795430.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Han YN, Ryu SY, Han BH (1990). "Antioxidant activity of resveratrol closely correlates with its monoamine oxidase-A inhibitory activity". Archives of Pharmacal Research. 13 (2): 132–135. doi:10.1007/BF02857789.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tsai RY, Chou KY, Shen CH, Chien CC, Tsai WY, Huang YN, Tao PL, Lin YS, Wong CS (October 2012). "Resveratrol regulates N-methyl-D-aspartate receptor expression and suppresses neuroinflammation in morphine-tolerant rats". Anesth. Analg. 115 (4): 944–52. doi:10.1213/ANE.0b013e31825da0fb. PMID 22713680.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA (March 2013). "Evidence for a common mechanism of SIRT1 regulation by allosteric activators". Science. 339 (6124): 1216–9. doi:10.1126/science.1231097. PMC 3799917. PMID 23471411.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM (August 2002). "Inhibition of cancer growth by resveratrol is related to its low bioavailability". Free Radic. Biol. Med. 33 (3): 387–98. doi:10.1016/S0891-5849(02)00911-5. PMID 12126761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Madhav NV, Shakya AK, Shakya P, Singh K (November 2009). "Orotransmucosal drug delivery systems: a review". J Control Release. 140 (1): 2–11. doi:10.1016/j.jconrel.2009.07.016. PMID 19665039.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Ansari KA, Vavia PR, Trotta F, Cavalli R (March 2011). "Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study". AAPS PharmSciTech. 12 (1): 279–86. doi:10.1208/s12249-011-9584-3. PMC 3066340. PMID 21240574.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shojaei AH (1998). "Buccal mucosa as a route for systemic drug delivery: a review". J Pharm Pharm Sci. 1 (1): 15–30. PMID 10942969.
- ^ Santos AC, Veiga F, Ribeiro AJ (August 2011). "New delivery systems to improve the bioavailability of resveratrol". Expert Opin Drug Deliv. 8 (8): 973–90. doi:10.1517/17425247.2011.581655. PMID 21668403.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK (December 2004). "High absorption but very low bioavailability of oral resveratrol in humans". Drug Metab. Dispos. 32 (12): 1377–82. doi:10.1124/dmd.104.000885. PMID 15333514.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Clinical trial number NCT00920556 for "A Clinical Study to Assess the Safety and Activity of SRT501 Alone or in Combination With Bortezomib in Patients With Multiple Myeloma" at ClinicalTrials.gov
- ^ la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron DW (July 2010). "Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects". Clin Pharmacokinet. 49 (7): 449–54. doi:10.2165/11531820-000000000-00000. PMID 20528005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP (July 2002). "Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model". J. Pharmacol. Exp. Ther. 302 (1): 369–73. doi:10.1124/jpet.102.033340. PMID 12065739.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice-Evans C, Spencer JP (July 2006). "Distribution of [3H]trans-resveratrol in rat tissues following oral administration". Br. J. Nutr. 96 (1): 62–70. doi:10.1079/BJN20061810. PMID 16869992.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB (December 2002). "Human, rat, and mouse metabolism of resveratrol". Pharm. Res. 19 (12): 1907–14. doi:10.1023/A:1021414129280. PMID 12523673.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang LX, Heredia A, Song H, Zhang Z, Yu B, Davis C, Redfield R (October 2004). "Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity". J Pharm Sci. 93 (10): 2448–57. doi:10.1002/jps.20156. PMID 15349955.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wenzel E, Somoza V (May 2005). "Metabolism and bioavailability of trans-resveratrol". Mol Nutr Food Res. 49 (5): 472–81. doi:10.1002/mnfr.200500010. PMID 15779070.
- ^ a b c Goldberg DM, Yan J, Soleas GJ (February 2003). "Absorption of three wine-related polyphenols in three different matrices by healthy subjects". Clin. Biochem. 36 (1): 79–87. doi:10.1016/S0009-9120(02)00397-1. PMID 12554065.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi PP, Fogliano V, Marchelli R (May 2005). "Bioavailability of trans-resveratrol from red wine in humans". Mol Nutr Food Res. 49 (5): 495–504. doi:10.1002/mnfr.200500002. PMID 15830336.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Corder R, Mullen W, Khan NQ, Marks SC, Wood EG, Carrier MJ, Crozier A (November 2006). "Oenology: red wine procyanidins and vascular health". Nature. 444 (7119): 566. doi:10.1038/444566a. PMID 17136085.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM (October 2000). "Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta". Endocrinology. 141 (10): 3657–67. doi:10.1210/en.141.10.3657. PMID 11014220.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Levi F, Pasche C, Lucchini F, Ghidoni R, Ferraroni M, La Vecchia C (April 2005). "Resveratrol and breast cancer risk". Eur. J. Cancer Prev. 14 (2): 139–42. doi:10.1097/00008469-200504000-00009. PMID 15785317.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Garvin S, Ollinger K, Dabrosin C (January 2006). "Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo". Cancer Lett. 231 (1): 113–22. doi:10.1016/j.canlet.2005.01.031. PMID 16356836.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bråkenhielm E, Cao R, Cao Y (August 2001). "Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes". FASEB J. 15 (10): 1798–800. doi:10.1096/fj.01-0028fje. PMID 11481234.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Leone S, Cornetta T, Basso E, Cozzi R (September 2010). "Resveratrol induces DNA double-strand breaks through human topoisomerase II interaction". Cancer Lett. 295 (2): 167–72. doi:10.1016/j.canlet.2010.02.022. PMID 20304553.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jo JY, Gonzalez de Mejia E, Lila MA (March 2006). "Catalytic inhibition of human DNA topoisomerase II by interactions of grape cell culture polyphenols". Journal of Agricultural and Food Chemistry. 54 (6): 2083–7. doi:10.1021/jf052700z. PMID 16536579.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Paolini M, Sapone A, Valgimigli L (June 2003). "Avoidance of bioflavonoid supplements during pregnancy: a pathway to infant leukemia?". Mutat. Res. 527 (1–2): 99–101. doi:10.1016/S0027-5107(03)00057-5. PMID 12787918.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alcaín FJ, Villalba JM (April 2009). "Sirtuin activators". Expert Opin Ther Pat. 19 (4): 403–14. doi:10.1517/13543770902762893. PMID 19441923.
- ^ Robb EL, Page MM, Wiens BE, Stuart JA (March 2008). "Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD". Biochem. Biophys. Res. Commun. 367 (2): 406–12. doi:10.1016/j.bbrc.2007.12.138. PMID 18167310.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Radák, Zsolt (2000). Free radicals in exercise and aging. Champaign, IL: Human Kinetics. p. 39. ISBN 978-0-88011-881-1.
- ^ Macmillan-Crow LA, Cruthirds DL (April 2001). "Invited review: manganese superoxide dismutase in disease". Free Radic. Res. 34 (4): 325–36. doi:10.1080/10715760100300281. PMID 11328670.
- ^ Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, Oberley LW (March 2003). "The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma". Cancer Res. 63 (6): 1297–303. PMID 12649190.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Mounting evidence shows red wine antioxidant kills cancer" (Press release). University of Rochester Medical Center. March 26, 2008. Retrieved August 10, 2010.
- ^ Sun J, Folk D, Bradley TJ, Tower J (June 2002). "Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster". Genetics. 161 (2): 661–72. PMC 1462135. PMID 12072463.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E (March 2007). "Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase". Neurobiol Learn Mem. 87 (3): 372–84. doi:10.1016/j.nlm.2006.10.003. PMC 1847321. PMID 17129739.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wong GH (May 1995). "Protective roles of cytokines against radiation: induction of mitochondrial MnSOD". Biochim. Biophys. Acta. 1271 (1): 205–9. doi:10.1016/0925-4439(95)00029-4. PMID 7599209.
- ^ Stefani M, Markus MA, Lin RC, Pinese M, Dawes IW, Morris BJ (October 2007). "The effect of resveratrol on a cell model of human aging". Annals of the New York Academy of Sciences. 1114: 407–18. doi:10.1196/annals.1396.001. PMID 17804521.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (March 2004). "Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase". Science. 303 (5666): 2011–5. doi:10.1126/science.1094637. PMID 14976264.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (September 2002). "Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress". Nature. 419 (6904): 316–21. doi:10.1038/nature01036. PMID 12239572.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khan MA, Chen HC, Wan XX, Tania M, Xu AH, Chen FZ, Zhang DZ (March 2013). "Regulatory effects of resveratrol on antioxidant enzymes: a mechanism of growth inhibition and apoptosis induction in cancer cells". Mol Cells. 35 (3): 219–25. doi:10.1007/s10059-013-2259-z. PMID 23456297.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leiro J, Arranz JA, Fraiz N, Sanmartín ML, Quezada E, Orallo F (February 2005). "Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling". Int. Immunopharmacol. 5 (2): 393–406. doi:10.1016/j.intimp.2004.10.006. PMID 15652768.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chun YJ, Kim MY, Guengerich FP (August 1999). "Resveratrol is a selective human cytochrome P450 1A1 inhibitor". Biochem. Biophys. Res. Commun. 262 (1): 20–4. doi:10.1006/bbrc.1999.1152. PMID 10448061.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schwarz D, Roots I (April 2003). "In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1". Biochem. Biophys. Res. Commun. 303 (3): 902–7. doi:10.1016/S0006-291X(03)00435-2. PMID 12670496.
- ^ a b Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellón EA (2007). "Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines". J. Androl. 28 (2): 282–93. doi:10.2164/jandrol.106.000968. PMID 17050787.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Faber AC, Chiles TC (December 2006). "Resveratrol induces apoptosis in transformed follicular lymphoma OCI-LY8 cells: evidence for a novel mechanism involving inhibition of BCL6 signaling". Int. J. Oncol. 29 (6): 1561–6. PMID 17088997.
- ^ Riles WL, Erickson J, Nayyar S, Atten MJ, Attar BM, Holian O (September 2006). "Resveratrol engages selective apoptotic signals in gastric adenocarcinoma cells". World J. Gastroenterol. 12 (35): 5628–34. PMID 17007014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sareen D, van Ginkel PR, Takach JC, Mohiuddin A, Darjatmoko SR, Albert DM, Polans AS (September 2006). "Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells". Invest. Ophthalmol. Vis. Sci. 47 (9): 3708–16. doi:10.1167/iovs.06-0119. PMID 16936077.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY (August 2006). "Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells". Mol. Cancer Ther. 5 (8): 2034–42. doi:10.1158/1535-7163.MCT-06-0216. PMID 16928824.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N (May 2006). "Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3'-kinase/Akt pathway and Bcl-2 family proteins". Mol. Cancer Ther. 5 (5): 1335–41. doi:10.1158/1535-7163.MCT-05-0526. PMID 16731767.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cao Y, Fu ZD, Wang F, Liu HY, Han R (June 2005). "Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants". J Asian Nat Prod Res. 7 (3): 205–13. doi:10.1080/10286020410001690190. PMID 15621628.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hung LM, Chen JK, Huang SS, Lee RS, Su MJ (August 2000). "Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes". Cardiovasc. Res. 47 (3): 549–55. doi:10.1016/S0008-6363(00)00102-4. PMID 10963727.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C (April 2005). "Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons". Nat. Genet. 37 (4): 349–50. doi:10.1038/ng1534. PMID 15793589.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG (March 2005). "Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol". Am. J. Physiol. Heart Circ. Physiol. 288 (3): H1131–8. doi:10.1152/ajpheart.00763.2004. PMID 15498824.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM (October 2001). "Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models". Cancer Res. 61 (20): 7456–63. PMID 11606380.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang Y, Lee KW, Chan FL, Chen S, Leung LK (July 2006). "The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells". Toxicol. Sci. 92 (1): 71–7. doi:10.1093/toxsci/kfj190. PMID 16611627.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I (March 2008). "Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells". Am. J. Physiol. Lung Cell Mol. Physiol. 294 (3): L478–88. doi:10.1152/ajplung.00361.2007. PMID 18162601.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ghosh HS, McBurney M, Robbins PD (2010). Blagosklonny, Mikhail V. (ed.). "SIRT1 negatively regulates the mammalian target of rapamycin". PLoS ONE. 5 (2): e9199. doi:10.1371/journal.pone.0009199. PMC 2821410. PMID 20169165.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, Madeo F, Kroemer G (December 2009). "Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol". Aging (Albany NY). 1 (12): 961–70. PMC 2815753. PMID 20157579.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tennen RI, Michishita-Kioi E, Chua KF (February 2012). "Finding a target for resveratrol". Cell. 148 (3): 387–9. doi:10.1016/j.cell.2012.01.032. PMID 22304906.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH (February 2012). "Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases". Cell. 148 (3): 421–33. doi:10.1016/j.cell.2012.01.017. PMC 3431801. PMID 22304913.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kenichi Mizutani, Katsumi Ikeda, Yukio Yamori (2000). "Resveratrol Inhibits AGEs-Induced Proliferation and Collagen Synthesis Activity in Vascular Smooth Muscle Cells from Stroke-Prone Spontaneously Hypertensive Rats". Biochemical and Biophysical Research Communications. 274 (1): 61–67. doi:10.1006/bbrc.2000.3097. PMID 10903896.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Mattivi F, Reniero F, Korhammer S (1995). "Isolation, characterization, and evolution in red wine vinification of resveratrol monomers". Journal of Agricultural and Food Chemistry. 43 (7): 1820–3. doi:10.1021/jf00055a013.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lamuela-Raventos RM, Romero-Perez AI, Waterhouse AL, de la Torre-Boronat MC (1995). "Direct HPLC Analysis of cis- and trans-Resveratrol and Piceid Isomers in Spanish Red Vitis vinifera Wines". Journal of Agricultural and Food Chemistry. 43 (2): 281–283. doi:10.1021/jf00050a003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon Vol. 84 No. 7 July 2007 Journal of Chemical Education 1159.
- ^ Yang, Ilseung; Kim, Eunha; Kang, Junhee; Han, Hyouksoo; Sul, Soohwan; Park, Seung Bum; Kim, Seong Keun (2012). "Photochemical generation of a new, highly fluorescent compound from non-fluorescent resveratrol". Chemical Communications. 48 (32): 3839–41. doi:10.1039/C2CC30940H. PMID 22436889.
- ^ Prokop J, Abrman P, Seligson AL, Sovak M (2006). "Resveratrol and its glycon piceid are stable polyphenols". J Med Food. 9 (1): 11–4. doi:10.1089/jmf.2006.9.11. PMID 16579722.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bertelli AA, Gozzini A, Stradi R, Stella S, Bertelli A (1998). "Stability of resveratrol over time and in the various stages of grape transformation". Drugs Exp Clin Res. 24 (4): 207–11. PMID 10051967.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sharan, S.; Nagar, S. (2013). "Pulmonary Metabolism of Resveratrol: In Vitro and in Vivo Evidence". Drug Metabolism and Disposition. 41 (5): 1163–9. doi:10.1124/dmd.113.051326. PMC 3629805. PMID 23474649.
- ^ Schröder G, Brown JW, Schröder J (February 1988). "Molecular analysis of resveratrol synthase. cDNA, genomic clones and relationship with chalcone synthase". Eur. J. Biochem. 172 (1): 161–9. doi:10.1111/j.1432-1033.1988.tb13868.x. PMID 2450022.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cichewicz RH, Kouzi SA, Hamann MT (January 2000). "Dimerization of resveratrol by the grapevine pathogen Botrytis cinerea". J. Nat. Prod. 63 (1): 29–33. doi:10.1021/np990266n. PMID 10650073.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cichewicz RH, Kouzi SA (October 1998). "Biotransformation of resveratrol to piceid by Bacillus cereus". J. Nat. Prod. 61 (10): 1313–4. doi:10.1021/np980139b. PMID 9784180.
- ^ Favaron, F.; Lucchetta, M.; Odorizzi, S.; Pais da Cunha, A.T.; Sella, L. (2009). "The role of grape polyphenols on trans-resveratrol activity against Botrytis cinerea and of fungal laccase on the solubility of putative grape PR proteins". Journal of Plant Pathology. 91 (3): 579–88. doi:10.4454/jpp.v91i3.549.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ Gatto P, Vrhovsek U, Muth J, Segala C, Romualdi C, Fontana P, Pruefer D, Stefanini M, Moser C, Mattivi F, Velasco R (December 2008). "Ripening and genotype control stilbene accumulation in healthy grapes". Journal of Agricultural and Food Chemistry. 56 (24): 11773–85. doi:10.1021/jf8017707. PMID 19032022.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Roy, H., Lundy, S., Resveratrol, Pennington Nutrition Series, 2005 No. 7
- ^ a b c d e LeBlanc, Mark Rene (13 December 2005). "Cultivar, Juice Extraction, Ultra Violet Irradiation and Storage Influence the Stilbene Content of Muscadine Grapes (Vitis Rotundifolia Michx.)". Retrieved 2007-08-15.
- ^ Gu X, Creasy L, Kester A, Zeece M (August 1999). "Capillary electrophoretic determination of resveratrol in wines". Journal of Agricultural and Food Chemistry. 47 (8): 3223–7. doi:10.1021/jf981211e. PMID 10552635.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mattivi F (June 1993). "Solid phase extraction of trans-resveratrol from wines for HPLC analysis". Z Lebensm Unters Forsch. 196 (6): 522–5. doi:10.1007/BF01201331. PMID 8328217.
- ^ Ector BJ, Magee JB, Hegwood CP, Coign MJ (1996). "Resveratrol Concentration in Muscadine Berries, Juice, Pomace, Purees, Seeds, and Wines". American Journal of Enology and Viticulture. 47 (1): 57–62.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G (August 2003). "Phenolic content and antioxidant capacity of muscadine grapes". Journal of Agricultural and Food Chemistry. 51 (18): 5497–503. doi:10.1021/jf030113c. PMID 12926904.
Contrary to previous results, ellagic acid and not resveratrol was the major phenolic in muscadine grapes. The HPLC solvent system used coupled with fluorescence detection allowed separation of ellagic acid from resveratrol and detection of resveratrol." "[T]rans-resveratrol had the lowest concentrations of the detected phenolics, ranging from not detected in two varieties to 0.2 mg/ 100 g of FW (Tables 1 and 2). Our result for resveratrol differed from previous results [Ector et al., 1996] indicating high concentrations. These researchers apparently were not able to separate ellagic acid from resveratrol with UV detection alone.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hudson TS, Hartle DK, Hursting SD, Nunez NP, Wang TT, Young HA, Arany P, Green JE (September 2007). "Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms". Cancer Res. 67 (17): 8396–405. doi:10.1158/0008-5472.CAN-06-4069. PMID 17804756.
MSKE [muscadine grape skin extract] does not contain significant quantities of resveratrol and differs from MSEE. To determine whether MSKE contains significant levels of resveratrol and to compare the chemical content of MSKE (skin) with MSEE (seed), HPLC analyses were done. As depicted in Supplementary Fig. S1A and B, MSKE does not contain significant amounts of resveratrol (<1 ?g/g by limit of detection).
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang KH, Lai YH, Chang JC, Ko TF, Shyu SL, Chiou RY (January 2005). "Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable". Journal of Agricultural and Food Chemistry. 53 (2): 242–6. doi:10.1021/jf048804b. PMID 15656656.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stewart JR, Artime MC, O'Brian CA (July 2003). "Resveratrol: a candidate nutritional substance for prostate cancer prevention". J. Nutr. 133 (7 Suppl): 2440S–2443S. PMID 12840221.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Hurst WJ, Glinski JA, Miller KB, Apgar J, Davey MH, Stuart DA (September 2008). "Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products". Journal of Agricultural and Food Chemistry. 56 (18): 8374–8. doi:10.1021/jf801297w. PMID 18759443.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mozzon M (1996). "Resveratrol content in some Tuscan wines". Ital. J. Food Sci. 8 (2). Chiriotti, Pinerolo, ITALIE: 145–52. INIST 3123149.
- ^ a b Stervbo U, Vang O, Bonnesen C (2007). "A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine". Food Chemistry. 101 (2): 449–57. doi:10.1016/j.foodchem.2006.01.047.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Higdon J, Drake VJ, Steward WP (May 2008). "Resveratrol". Micronutrient Information Center. Linus Pauling Institute.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ <Please add first missing authors to populate metadata.> (1999). "Resveratrol In Peanuts". Kids Food for Thought. 1 (4). The Peanut Institute. Archived from the original on August 24, 2000.[unreliable source?]
- ^ Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB (January 2002). "An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine". Journal of Agricultural and Food Chemistry. 50 (3): 431–5. doi:10.1021/jf010812u. PMID 11804508.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lyons MM, Yu C, Toma RB, Cho SY, Reiboldt W, Lee J, van Breemen RB (September 2003). "Resveratrol in raw and baked blueberries and bilberries". Journal of Agricultural and Food Chemistry. 51 (20): 5867–70. doi:10.1021/jf034150f. PMID 13129286.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rimas A (2006-12-11). "His research targets the aging process". The Boston Globe.
- ^ Stipp D (2007-01-19). "Can red wine help you live forever?". Fortune magazine.
- ^ a b Seward ZM (2006-11-30). "Quest for youth drives craze for 'wine' pills". The Wall Street Journal.
- ^ "Caution urged with resveratrol". United Press International. November 30, 2006-11-30.
{{cite news}}: Check date values in:|date=(help) - ^ Aleccia J (2008-04-22). "Longevity quest moves slowly from lab to life". MSNBC.
- ^ Gocze T (2008-09-08). "Japanese Knotweed a Resilient Invader". Bangor Daily News.
- ^ Weintraub A (2009-07-29). "Resveratrol: The Hard Sell on Anti-Aging". Bloomberg Businessweek.
- ^ Kim JK, Kim M, Cho SG, Kim MK, Kim SW, Lim YH (June 2010). "Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition". J. Ind. Microbiol. Biotechnol. 37 (6): 631–7. doi:10.1007/s10295-010-0722-9. PMID 20411402.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Antibacterial phenolics from Boswellia dalzielii. Alemika Taiwo E, Onawunmi Grace O and Olugbade Tiwalade O, Nigerian Journal of Natural Products and Medicines, 2006 (abstract)[dead link]
External links
- Félicien Breton (2008). "Resveratrol and polyphenols in wines".
- CTD's Resveratrol page from the Comparative Toxicogenomics Database
- U.S. National Library of Medicine: Drug Information Portal – Resveratrol
- Detailed Micro-Nutrient information on Resveratrol from the Linus Pauling Institute
- Resveratrol: Don't Buy the Hype
- Stay young on red wine drugs? Think again