revise preparative details per SmCl3 and GdCl3 articles |
Project Osprey (talk | contribs) →Uses: Silylamide prep |
||
| Line 65: | Line 65: | ||
==Uses== |
==Uses== |
||
Europium(III) chloride can be used for the preparation of [[europium(II) chloride]] by reduction in a gold boat using [[hydrogen]] gas while heating slowly to 700 °C. The anhydrous [[chloride]] may also be used to prepare [[organometallic]] compounds of europium, such as bis(pentamethylcyclopentadienyl)europium(II) complexes.<ref>{{cite journal | doi = 10.1021/ic50212a031 | year = 1980 | author = Tilley, T. Don | journal = Inorganic Chemistry | volume = 19 | pages = 2999 | title = Divalent lanthanide chemistry. Bis (pentamethylcyclopentadienyl) europium(II) and -ytterbium(II) derivatives: crystal structure of bis (pentamethylcyclopentadienyl) (tetrahydrofuran ytterbium(II) -hemitoluene at 176 K | last2 = Andersen | first2 = Richard A. | last3 = Spencer | first3 = Brock | last4 = Ruben | first4 = Helena | last5 = Zalkin | first5 = Allan | last6 = Templeton | first6 = David H. | issue = 10}}</ref><ref>{{cite journal | doi = 10.1021/om00138a001 | year = 1986 | author = Evans, William J. | journal = Organometallics | volume = 5 | pages = 1285 | title = Synthesis and x-ray crystal structure of bis(pentamethylcyclopentadienyl) complexes of samarium and europium: (C<sub>5</sub>Me<sub>5</sub>)<sub>2</sub>Sm and (C<sub>5</sub>Me<sub>5</sub>)<sub>2</sub>Eu | last2 = Hughes | first2 = Laura A. | last3 = Hanusa | first3 = Timothy P. | issue = 7}}</ref> Europium(III) chloride can be used as a starting point for the preparation of other [[europium]] [[salt]]s. |
Europium(III) chloride can be used for the preparation of [[europium(II) chloride]] by reduction in a gold boat using [[hydrogen]] gas while heating slowly to 700 °C. The anhydrous [[chloride]] may also be used to prepare [[organometallic]] compounds of europium, such as bis(pentamethylcyclopentadienyl)europium(II) complexes.<ref>{{cite journal | doi = 10.1021/ic50212a031 | year = 1980 | author = Tilley, T. Don | journal = Inorganic Chemistry | volume = 19 | pages = 2999 | title = Divalent lanthanide chemistry. Bis (pentamethylcyclopentadienyl) europium(II) and -ytterbium(II) derivatives: crystal structure of bis (pentamethylcyclopentadienyl) (tetrahydrofuran ytterbium(II) -hemitoluene at 176 K | last2 = Andersen | first2 = Richard A. | last3 = Spencer | first3 = Brock | last4 = Ruben | first4 = Helena | last5 = Zalkin | first5 = Allan | last6 = Templeton | first6 = David H. | issue = 10}}</ref><ref>{{cite journal | doi = 10.1021/om00138a001 | year = 1986 | author = Evans, William J. | journal = Organometallics | volume = 5 | pages = 1285 | title = Synthesis and x-ray crystal structure of bis(pentamethylcyclopentadienyl) complexes of samarium and europium: (C<sub>5</sub>Me<sub>5</sub>)<sub>2</sub>Sm and (C<sub>5</sub>Me<sub>5</sub>)<sub>2</sub>Eu | last2 = Hughes | first2 = Laura A. | last3 = Hanusa | first3 = Timothy P. | issue = 7}}</ref> Europium(III) chloride can be used as a starting point for the preparation of other [[europium]] [[salt]]s. |
||
Anhydrous europium(III) chloride can be converted to the corresponding [[metal bis(trimethylsilyl)amides|metal bis(trimethylsilyl)amide]] via a metathesis reaction with [[lithium bis(trimethylsilyl)amide]].<ref name="lan">>{{cite journal|last=Bradley|first=Donald C.|coauthors=Ghotra, Joginder S.; Hart, F. Alan|title=Low co-ordination numbers in lanthanide and actinide compounds. Part I. The preparation and characterization of tris{bis(trimethylsilyl)-amido}lanthanides|journal=Journal of the Chemical Society, Dalton Transactions|year=1973|issue=10|pages=1021|doi=10.1039/DT9730001021|url=http://pubs.rsc.org/en/content/articlelanding/1973/dt/dt9730001021}}</ref> The reaction is performed in [[THF]] and requires a period at reflux. Once formed, the product is separated from [[LiCl]] by exchanging the solvent for toluene, in which Eu(N(SiMe<sub>3</sub>)<sub>2</sub>)<sub>3</sub> is soluble but LiCl is not. |
|||
: Eu(Cl)<sub>3</sub> + 3 LiN(SiMe<sub>3</sub>)<sub>2</sub> → Eu(N(SiMe<sub>3</sub>)<sub>2</sub>)<sub>3</sub> + 3 LiCl + 3 butane |
|||
Eu(N(SiMe<sub>3</sub>)<sub>2</sub>)<sub>3</sub> is commonly used as a starting material for the more complicated [[coordination complex]]es, as Eu(Cl)<sub>3</sub> has either poor solubility or poor stability in common solvents. |
|||
==Bibliography== |
==Bibliography== |
||
Revision as of 21:20, 12 May 2013

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Europium(III) chloride
Europium trichloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.025 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| EuCl3 | |||
| Molar mass | 258.323 g/mol 366.41 g/mol (hexahydrate) | ||
| Melting point | 632 °C decomp. | ||
| Solubility in other solvents | Soluble | ||
| Related compounds | |||
| Structure | |||
| hexagonal (UCl3 type), hP8 | |||
| P63/m, No. 176 | |||
| Tricapped trigonal prismatic (nine-coordinate) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
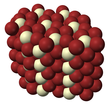
Europium(III) chloride is an inorganic compound with the formula EuCl3. Europium trichloride is a yellow solid which begins to decompose at or below its melting point to give at least some EuCl2. Being hygroscopic it rapidly absorbs water on exposure to moist air to form a white crystalline hexahydrate, EuCl3·6H2O. Simple rapid heating of the hydrate alone may cause small amounts of hydrolysis. Europium(III) chloride is soluble in water. When anhydrous, it is expected to be also highly soluble in ethanol (by analogy with SmCl3). It is nine-coordinate (trigonal prismatic), and it crystallises with the UCl3 structure.[1]
Preparation
SmCl3 is prepared by the "ammonium chloride" route, which involves the initial synthesis of (NH4)2[SmCl5]. This material can be prepared from the common starting materials at reaction temperatures of 230 °C from samarium oxide:[2]
- 10 NH4Cl + Eu2O3 → 2 (NH4)2[EuCl5] + 6 NH3 + 3 H2O
The pentachloride is then heated to 350-400 °C resulting in evolution of ammonium chloride and leaving a residue of the anhydrous trichloride:
- (NH4)2[EuCl5] → 2 NH4Cl2 + EuCl3
Uses
Europium(III) chloride can be used for the preparation of europium(II) chloride by reduction in a gold boat using hydrogen gas while heating slowly to 700 °C. The anhydrous chloride may also be used to prepare organometallic compounds of europium, such as bis(pentamethylcyclopentadienyl)europium(II) complexes.[3][4] Europium(III) chloride can be used as a starting point for the preparation of other europium salts.
Anhydrous europium(III) chloride can be converted to the corresponding metal bis(trimethylsilyl)amide via a metathesis reaction with lithium bis(trimethylsilyl)amide.[5] The reaction is performed in THF and requires a period at reflux. Once formed, the product is separated from LiCl by exchanging the solvent for toluene, in which Eu(N(SiMe3)2)3 is soluble but LiCl is not.
- Eu(Cl)3 + 3 LiN(SiMe3)2 → Eu(N(SiMe3)2)3 + 3 LiCl + 3 butane
Eu(N(SiMe3)2)3 is commonly used as a starting material for the more complicated coordination complexes, as Eu(Cl)3 has either poor solubility or poor stability in common solvents.
Bibliography
- Edelmann, F. T.; & Poremba, P. (1997). in: Synthetic Methods of Organometallic and Inorganic Chemistry (Herrmann, E. A., Ed.) Vol. 6. Stuttgart:Georg Thieme.
- Weast, R. C., ed. (1972). Handbook of Chemistry and Physics (53rd ed.). Cleveland, OH: Chemical Rubber Co.
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^
Meyer, G. (1989). "The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3". Inorganic Syntheses. 25: 146–150. doi:10.1002/9780470132562.ch35. ISBN 978-0-470-13256-2.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Tilley, T. Don; Andersen, Richard A.; Spencer, Brock; Ruben, Helena; Zalkin, Allan; Templeton, David H. (1980). "Divalent lanthanide chemistry. Bis (pentamethylcyclopentadienyl) europium(II) and -ytterbium(II) derivatives: crystal structure of bis (pentamethylcyclopentadienyl) (tetrahydrofuran ytterbium(II) -hemitoluene at 176 K". Inorganic Chemistry. 19 (10): 2999. doi:10.1021/ic50212a031.
- ^ Evans, William J.; Hughes, Laura A.; Hanusa, Timothy P. (1986). "Synthesis and x-ray crystal structure of bis(pentamethylcyclopentadienyl) complexes of samarium and europium: (C5Me5)2Sm and (C5Me5)2Eu". Organometallics. 5 (7): 1285. doi:10.1021/om00138a001.
- ^ >Bradley, Donald C. (1973). "Low co-ordination numbers in lanthanide and actinide compounds. Part I. The preparation and characterization of tris{bis(trimethylsilyl)-amido}lanthanides". Journal of the Chemical Society, Dalton Transactions (10): 1021. doi:10.1039/DT9730001021.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)