| |
| Names | |
|---|---|
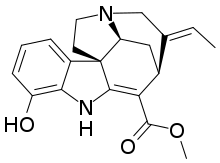
| IUPAC name
Methyl (19E)-12-hydroxy-2,16-didehydrocur-19-en-17-oate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H39NO7 | |
| Molar mass | 453.576 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Vinervine is a monoterpene indole alkaloid of the Vinca sub-group. It is a derivative of akuammicine, with one additional hydroxy (OH) group in the indole portion, hence it is also known as 12-hydroxyakuammicine.
History[edit]
The alkaloids are a large group of natural products which are classified according to the part-structure which members of a particular group contain. Vinervine is a monoterpene indole alkaloid of the Vinca sub-group which shares a common biosynthesis with other members, namely that they are derived from strictosidine.[1][2] It was first characterised in 1964[3] and the structures of closely related materials including akuammicine were confirmed in 1983.[4]
Natural occurrence[edit]

Vinervine is found in a variety of plants of the Apocynaceae family, including Vinca erecta,[3][5][6] Tabernaemontana divaricata[7][8] and several other flowering plants species that are native to Africa, Asia, and Europe.
Biosynthesis[edit]
As with other indole alkaloids, the biosynthesis of vinervine starts from the amino acid tryptophan. This is converted into strictosidine before further elaboration.[1]
Research[edit]
Plant metabolites have long been studied for their biological activity and alkaloids in particular are major subjects for ethnobotanical research.[9] However, vinervine has had little reported utility.[8][10][11]
See also[edit]
References[edit]
- ^ a b Dewick, Paul M (2002). Medicinal Natural Products. A Biosynthetic Approach. Second Edition. Wiley. pp. 350–359. ISBN 0-471-49640-5.
- ^ Saxton JE (1984). "Recent progress in the chemistry of indole alkaloids and mould metabolites". Natural Product Reports. 1: 21. doi:10.1039/NP9840100021.
- ^ a b Abdurakhimova N, Yuldashev PK, Yunusov, SY (1964). "Pseudokopsinine—a new alkaloid from aerial parts of Vinca erecta". Doklady Akademii Nauk SSSR (in Russian). 21 (2): 29–31.
- ^ Yagudaev MR (1983). "NMR investigation of alkaloids. IV. 13C NMR spectra and structures of norfluorocurarine, akuammicine, vincanidine, and vinervinine". Chemistry of Natural Compounds. 19 (2): 199–201. doi:10.1007/BF00580558. S2CID 28255077.
- ^ Yuldashev PK, Ubaev U, Kuchenkova MA, Yunusov SY (January 1965). "Structure of vincanidine and vinervine". Chemistry of Natural Compounds. 1 (1): 25–30. doi:10.1007/BF00571576. S2CID 27933208.
- ^ Kuchenkova MA, Yuldashev PK, Yunusov SY (1965). "Vinervine — A new alkaloid from the aboveground part of Vinca erecta". Bulletin of the Academy of Sciences, USSR Division of Chemical Science. 14 (12): 2119–2121. doi:10.1007/BF00845999.
- ^ Pawelka KH, Stöckigt J (April 1983). "Indole alkaloids from cell suspension cultures of Tabernaemontana divaricata and Tabernanthe iboga". Plant Cell Reports. 2 (2): 105–7. doi:10.1007/BF00270178. PMID 24257961. S2CID 23570705.
- ^ a b Pratchayasakul W, Pongchaidecha A, Chattipakorn N, Chattipakorn S (April 2008). "Ethnobotany & ethnopharmacology of Tabernaemontana divaricata". The Indian Journal of Medical Research. 127 (4): 317–35. PMID 18577786. S2CID 1119874.
- ^ Babiaka SB, Ntie-Kang F, Lifongo LL, Ndingkokhar B, Mbah JA, Yong JN (2015). "The chemistry and bioactivity of Southern African flora I: A bioactivity versus ethnobotanical survey of alkaloid and terpenoid classes". RSC Advances. 5 (54): 43242–43267. Bibcode:2015RSCAd...543242B. doi:10.1039/C5RA01912E.
- ^ Ghisalberti EL, Pennacchio M, Alexander E (1998). "Survey of Secondary Plant Metabolites with Cardiovascular Activity". Pharmaceutical Biology. 36 (4): 259. doi:10.1076/phbi.36.4.237.4583.
- ^ Heijden R, Jacobs D, Snoeijer W, Hallard D, Verpoorte R (2004). "The Catharanthus Alkaloids:Pharmacognosy and Biotechnology". Current Medicinal Chemistry. 11 (5): 607–628. doi:10.2174/0929867043455846. PMID 15032608.
Further reading[edit]
- Edwin Saxton J (15 September 2009). Indoles, Part 4: The Monoterpenoid Indole Alkaloids. John Wiley & Sons. ISBN 978-0-470-18844-6.