 | |
| Clinical data | |
|---|---|
| Other names | X4P-001; AMD-070 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
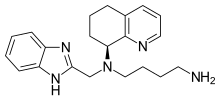
| Formula | C21H27N5 |
| Molar mass | 349.482 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mavorixafor (X4P-001)[1] is an orally bioavailable CXCR4 antagonist.[2] Developed by X4 Pharmaceuticals, it has been tested in clinical trials for WHIM syndrome,[3] melanoma,[2] and renal cell carcinoma.[4]
References[edit]
- ^ Liu, Weiyuan; Bi, Siju; Tian, Ting; Zhou, Ting; Lin, Kuaile; Zhou, Weicheng (17 June 2022). "A Novel and Practical Synthesis of Mavorixafor". Organic Process Research & Development. 26 (6): 1831–1836. doi:10.1021/acs.oprd.2c00076. S2CID 249557115.
- ^ a b Andtbacka, Robert H.I.; Wang, Yan; Pierce, Robert H.; Campbell, Jean S.; Yushak, Melinda; Milhem, Mohammed; Ross, Merrick; Niland, Katie; Arbeit, Robert D.; Parasuraman, Sudha; Bickley, Kris; Yeung, Cecilia CS; Aicher, Lauri D.; Smythe, Kimberly S.; Gan, Lu (31 August 2022). "Mavorixafor, an Orally Bioavailable CXCR4 Antagonist, Increases Immune Cell Infiltration and Inflammatory Status of Tumor Microenvironment in Patients with Melanoma". Cancer Research Communications. 2 (8): 904–913. doi:10.1158/2767-9764.CRC-22-0090. PMC 10010370. PMID 36923305.
- ^ Dale, David C.; Alsina, Laia; Azar, Antoine; Badolato, Raffaele; Bhandari, Ashish; Belschner, Andrea; Bertrand, Yves; Cadavid, Diego; Dickerson, Kathryn E.; Ezra, Navid; Hasle, Henrik; Hoffman, Harold; Jiang, Honghua; Kang, Hyoung Jin; Kiani-Alikhan, Sorena; Kuijpers, Taco W.; Kulagin, Aleksandr D.; Langguth, Daman; Levin, Carina; MacLeod, Richard; Neth, Olaf; Peake, Jane; Rodina, Yulia; Sharathkumar, Anjali; Shcherbina, Anna; Tang, Weihua; Vossen, Matthias G.; Donadieu, Jean (5 November 2021). "Global Phase 3, Randomized, Placebo-Controlled Trial with Open-Label Extension Evaluating the Oral CXCR4 Antagonist Mavorixafor in Patients with WHIM Syndrome (4WHIM): Trial Design and Enrollment". Blood. 138 (Supplement 1): 4310. doi:10.1182/blood-2021-153346.
- ^ Choueiri, Toni K.; Atkins, Michael B.; Rose, Tracy L.; Alter, Robert S.; Ju, Yawen; Niland, Katie; Wang, Yan; Arbeit, Robert; Parasuraman, Sudha; Gan, Lu; McDermott, David F. (August 2021). "A phase 1b trial of the CXCR4 inhibitor mavorixafor and nivolumab in advanced renal cell carcinoma patients with no prior response to nivolumab monotherapy". Investigational New Drugs. 39 (4): 1019–1027. doi:10.1007/s10637-020-01058-2. PMID 33507454. S2CID 231746027.