No edit summary |
Expand pregnancy. |
||
| (One intermediate revision by the same user not shown) | |||
| Line 33: | Line 33: | ||
=== Pregnancy === |
=== Pregnancy === |
||
Pregnant women who wish to quit smoking but are unable, are left with few options.<ref name=LiuLugo2018/> As [[nicotine replacement therapy|nicotine replacement products]] is often ineffective for quitting smoking, pregnant women turn to alternatives such as heat-not-burn tobacco.<ref name=LiSaad2018/> There is no information available on potential impact of maternal inhalation of heat-not-burn tobacco emissions during pregnancy on fetal outcomes.<ref name=LiSaad2018/> The risk to the fetus from heat-not-burn tobacco products during pregnancy is hard to quantify.<ref name=COT2017/> Although the risk to the fetus is probably less than smoking during pregnancy, the Committee on Toxicity recommends to completely stop smoking.<ref name=COT2017/> |
Pregnant women who wish to quit smoking but are unable, are left with few options.<ref name=LiuLugo2018/> As [[nicotine replacement therapy|nicotine replacement products]] is often ineffective for quitting smoking, pregnant women turn to alternatives such as heat-not-burn tobacco.<ref name=LiSaad2018/> There is no information available on potential impact of maternal inhalation of heat-not-burn tobacco emissions during pregnancy on fetal outcomes.<ref name=LiSaad2018/> The risk to the fetus from heat-not-burn tobacco products during pregnancy is hard to quantify.<ref name=COT2017/> Although the risk to the fetus is probably less than smoking during pregnancy, the Committee on Toxicity recommends to completely stop smoking.<ref name=COT2017/> Nicotine is harmful to the infant and the growing adolescent brain.<ref name=JenssenWalley2017/> |
||
Nicotine can lead to vasoconstriction of uteroplacental vessels, reducing the delivery of both nutrients and oxygen to the fetus.<ref name=LiSaad2018/> As a result, nutrition is re-distributed to prioritize vital organs, such as the heart and the brain, at the cost of less vital organs, such as the liver, kidneys, adrenal glands, and pancreas, leading to underdevelopment and functional disorders later in life.<ref name=LiSaad2018/> Animal research using maternal nicotine exposure showed a direct adverse impact on pancreas development by reducing endocrine pancreatic islet size and number.<ref name=LiSaad2018/> This was accompanied by a decrease in gene expression of specific transcription factors and blood glucose regulating hormones such as insulin and glucagon.<ref name=LiSaad2018/> As a result, rats exhibited significant pancreatic dysfunction and glucose intolerance.<ref name=LiSaad2018/> Moreover, several animal studies have reported insulin resistance in adult offspring due to maternal nicotine exposure.<ref name=LiSaad2018/> In animal models, nicotine has also been shown to activate nicotine acetylcholine receptors (nAChR) in the brain, which are important in regulating brain development.<ref name=LiSaad2018/> Nicotine exposure during the first trimester of pregnancy (2 mg/kg/d) leads to structural changes in the hippocampus and somatosensory cortex in rats.<ref name=LiSaad2018/> |
|||
== Construction == |
== Construction == |
||
Revision as of 05:13, 20 January 2019
A heat-not-burn tobacco product[notes 1] heats up tobacco using a battery-powered heating-system.[4] As it starts to heat the tobacco, it generates an aerosol that contains nicotine and other chemicals, that is inhaled.[4] They may or may not generate smoke.[10] They contain nicotine, which is the reason these products are addictive.[4] They also contain additives not found in tobacco, and are frequently flavored.[4] It heats tobacco leaves at a lower temperature than traditional cigarettes,[11] which is about 250–350 °C (around 500 °F.[12]).[13] These products provide some of the behavioral aspects of smoking.[14] There are different types of heat-not-burn tobacco products.[15] One type uses an embedded heat source; another type uses an external heat source; another one uses a heated sealed chamber; to deliver nicotine using tobacco leaf.[15] Some use product-specific customized cigarettes.[4] They are not electronic cigarettes.[4] They can overlap with e-cigarettes such as a combination of an e-cigarette and a heat-not-burn tobacco product, for the use of tobacco or e-liquid.[15]
A 2016 World Health Organization report found no compelling evidence has been presented for the claims of lowered risk or health benefits compared with traditional cigarettes, which are based on industry-funded research for these products.[16] A 2018 Public Health England (PHE) report states that the evidence indicates that heat-not-burn tobacco products may be much safer than traditional cigarettes but less safe than e-cigarettes.[17] The aerosol contains levels of nicotine and cancer-causing chemicals comparable to regular cigarettes.[1] Another source found, some of the substances inhaled from using these products are carcinogens.[18] Although heat-not-burn tobacco products are probably less dangerous than smoking, it would be better for smokers to completely stop, according to the Committee on Toxicity.[18] There is a lack of evidence on the possible effects of second-hand exposure.[4] The limited evidence on air emissions from the use of heat-not-burn tobacco products indicates that toxic exposure from these products is greater than that of e-cigarettes.[19] There is insufficient evidence on the efficacy of such products on quitting smoking.[11]
As early as the 1960s, the tobacco companies developed alternative tobacco products with the goal of supplementing the cigarette market with products.[20] Heat-not-burn tobacco products first came to market in 1988, however they were not a commercial success.[12] There has been a global decline in tobacco consumption that, if continued, will negatively impact the tobacco industry's profits.[20] This decline led the industry to invent and market new products, such as heat-not-burn tobacco products.[20] Smokers regularly reported heat-not-burn tobacco product use to be less satisfying than smoking a cigarette.[19] These products have been introduced by large tobacco companies.[21] The introduction of the latest generation of heat-not-burn tobacco products appears to be the latest chapter in the decades-old tobacco industry strategy of working to create partnerships with governments and health advocates, presenting these alleged 'harm reduction' products as an option to address the tobacco epidemic.[20] Current smoking bans may not have been extended to include such products.[22]
Health effects

As of December 2017[update], it is impossible to quantify the health risk from using these products.[18] There is very limited information available on their health effects.[18] It is not clear what the adverse effects are in the short-term.[23] The long-term effects are unclear.[24] A 2016 Cochrane review found that it was unclear whether using an electronically-heated cigarette smoking system instead of traditional cigarettes would "substantially alter the risk of harm".[6] There is a lack of long-term studies.[13] There are different kinds of heat-not-burn tobacco products available and therefore the effects each kind produces will vary.[25] This creates a challenge for researchers.[25] It is not known how users evaluate the safety of these products.[26] Among those who have tried such products, approximately 50% believe they are safer than traditional cigarettes and the other 50% believe they are just as unsafe as traditional cigarettes.[26]
A 2016 World Health Organization report stated claims of lowered risk or health benefits for heat-not-burn tobacco products compared with traditional cigarettes are based on industry-funded research, but compelling independent research is not available to support these claims.[16] There is not enough research to provide a balanced assessment of their harm.[24] With an assorted range of electronic cigarettes devices in the UK, it is unclear whether heat-not-burn tobacco products will offer any favorable benefit as an another plausible harm reduction product.[17] A 2016 World Health Organization reported noted that some scientists believe that heat-not-burn tobacco products to be as dangerous as traditional cigarettes.[16] A 2018 Public Health England (PHE) report states that the evidence indicates that heat-not-burn tobacco products may be much safer than traditional cigarettes but less safe than e-cigarettes.[17] Although heat-not-burn tobacco products are probably less dangerous than smoking, it would be better for smokers to completely stop, according to the Committee on Toxicity in 2017.[18]
Action on Smoking and Health in the UK stated in 2016 that due to "the tobacco industry's long record of deceit" regarding the health risks involving smoking, it is important to conduct independent studies into the health effects of these products.[27]
Emissions
The aerosol contains levels of nicotine and cancer-causing chemicals comparable to regular cigarettes.[1] They contain comparable levels of many volatile organic compounds and greater amounts of the polycyclic aromatic hydrocarbon acenaphthene than regular cigarettes.[10] The substances in the emissions of traditional cigarettes such as tar, nicotine, carbonyl compounds (including acetaldehyde, acrolein, and formaldehyde), and nitrosamines are also found in emissions of heat-not-burn tobacco products.[28] A 2017 study found a 10% rise in carbon monoxide and formaldehyde air levels than compared to the background during heat-not-burn tobacco product use.[29] A 2017 study found heat-not-burn tobacco products generated emissions of particulate metals and organic compounds as well as aldehydes.[29] Research suggests that heat-not-burn tobacco products generate less concentrations of airborne contaminates in indoor places in comparison to a traditional cigarette.[29]
A 2018 PHE report found "Compared with cigarettes, heated tobacco products are likely to expose users and bystanders to lower levels of particulate matter and harmful and potentially harmful compounds (HPHC). The extent of the reduction found varies between studies."[25] They also noted that the evidence indicates that the levels of nicotine inhaled from heat-not-burn tobacco products is less than that of cigarette smoke.[30] Exposure to mutagenic and other harmful substances is lower than with traditional cigarettes.[13] However, reduced exposure to harmful substances does not mean that health risks are equally reduced.[13] Even low exposure increases the risks for cancers, stroke, and other cardiovascular diseases compared to non-smokers.[13] It is still unclear to what extent the reduced levels lead to lowered health risks.[13] Although lower emissions have been shown, lowering the risk to the smoker who transitions to heat-not-burn tobacco products has not been shown, as of 2018.[31] Heat-not-burn tobacco products do not reduce being exposed to nicotine or the risk for the potential addiction to nicotine, according to the Committee on Toxicity in 2017.[18] Some of the substances inhaled from using these products are carcinogens.[18]
The physiological changes, such as inflammation in multiple organ systems, energy metabolism, and carcinogenesis, in responses to heat-not-burn tobacco emissions has not been well characterized due to limited research in this area, especially in animal models.[11] A 2018 in vitro study suggested a less harmful pathophysiological response in human organotypic oral epithelial cultures when exposed to such emissions.[11] A 2016 animal study showed that heat-not-burn tobacco emissions did not increase surfactant lipids, surfactant proteins, surfactant metabolizing proteins, inflammatory eicosanoids and their metabolic enzymes, and several ceramide classes, compared with tobacco cigarette smoke-exposed mice.[11] A 2016 study found that even with reduced toxicants in heat-not-burn tobacco emissions, overuse (40 tobacco sticks per day) can still lead to eosinophilic pneumonia in humans.[11]
The impact on the overall population is unclear.[24] There is disagreement to the extent to which heat-not-burn tobacco products generate air emissions .[32] There is also disagreement regarding the composition of the air emissions.[32] There is a lack of evidence on the possible effects of second-hand exposure.[4] There is anticipated to be a reduced risk to bystanders where smokers were using heat-not-burn tobacco products instead of smoking.[18] The limited evidence on air emissions from the use of heat-not-burn tobacco products indicates that toxic exposure from these products is greater than that of e-cigarettes.[19]
Addiction and quitting
In 2017, Ministry of Health in New Zealand stated "There is limited information on product use, including whether smokers are likely to switch completely from tobacco smoking or use both types of product, as well as initiation by non-smokers (including young people)."[33] In 2017, the Committee on Toxicity stated "The Committees were concerned over the potential for non-smokers including children and young people, who would not otherwise start to smoke cigarettes, to take up using these products as they are not without risk."[34] Compared to not using heat-not-burn tobacco products, there is an increase in risk for non-smokers who begin using them.[34] A growing body of evidence shows that never-users of tobacco products, especially children and adolescents, could be susceptible to new products and that this could result in later use of traditional cigarettes.[1] In 2017, the Committee on Toxicity "Committees were particularly concerned for young people, who do not smoke, starting to use these products, due to the potential for longer exposure over the remainder of their lives compared to adults and to possible differences in sensitivity."[34]
They contain nicotine, which is the reason these products are addictive.[4] The nicotine content in the emissions of heat-not-burn tobacco products is in the same range as the nicotine emissions of traditional cigarettes, which suggests a comparable addictiveness and dependence potential.[13] Dual use among heat-not-burn tobacco regular users with combustible products is common.[35] As of July 2017, not many US adults had tried using a heat-not-burn tobacco product.[36] Nearly half of Italian IQOS users (45%) and over half of the people interested in IQOS (51%) are never smokers.[37] Therefore, such a product may represent, at least in Italy, a gateway for nicotine addiction among never smokers rather than a harm reduction substitution for current smokers.[37] In Germany, heat-not-burn tobacco product use is not common and is generally more frequent among smokers who have been educated longer and who make more money.[26] Use in Japan, where that have been sold since 2014, is much higher.[26] Use in Italy was 1.4% among the people and 3.1% among regular tobacco users.[26] Research demonstrated that users of heat-not-burn tobacco products is greater among women who smoke.[26]
They are commonly used instead of or along with combustible tobacco products.[26] There is insufficient evidence on the efficacy of heat-not-burn tobacco products on quitting smoking.[11] A 2018 World Health Organization report states that "Conclusions cannot yet be drawn about their ability to assist with quitting smoking (cessation), their potential to attract new youth tobacco users (gateway effect), or the interaction in dual use with other conventional tobacco products and e-cigarettes."[4]
Pregnancy
Pregnant women who wish to quit smoking but are unable, are left with few options.[37] As nicotine replacement products is often ineffective for quitting smoking, pregnant women turn to alternatives such as heat-not-burn tobacco.[11] There is no information available on potential impact of maternal inhalation of heat-not-burn tobacco emissions during pregnancy on fetal outcomes.[11] The risk to the fetus from heat-not-burn tobacco products during pregnancy is hard to quantify.[18] Although the risk to the fetus is probably less than smoking during pregnancy, the Committee on Toxicity recommends to completely stop smoking.[18] Nicotine is harmful to the infant and the growing adolescent brain.[1]
Nicotine can lead to vasoconstriction of uteroplacental vessels, reducing the delivery of both nutrients and oxygen to the fetus.[11] As a result, nutrition is re-distributed to prioritize vital organs, such as the heart and the brain, at the cost of less vital organs, such as the liver, kidneys, adrenal glands, and pancreas, leading to underdevelopment and functional disorders later in life.[11] Animal research using maternal nicotine exposure showed a direct adverse impact on pancreas development by reducing endocrine pancreatic islet size and number.[11] This was accompanied by a decrease in gene expression of specific transcription factors and blood glucose regulating hormones such as insulin and glucagon.[11] As a result, rats exhibited significant pancreatic dysfunction and glucose intolerance.[11] Moreover, several animal studies have reported insulin resistance in adult offspring due to maternal nicotine exposure.[11] In animal models, nicotine has also been shown to activate nicotine acetylcholine receptors (nAChR) in the brain, which are important in regulating brain development.[11] Nicotine exposure during the first trimester of pregnancy (2 mg/kg/d) leads to structural changes in the hippocampus and somatosensory cortex in rats.[11]
Construction
-
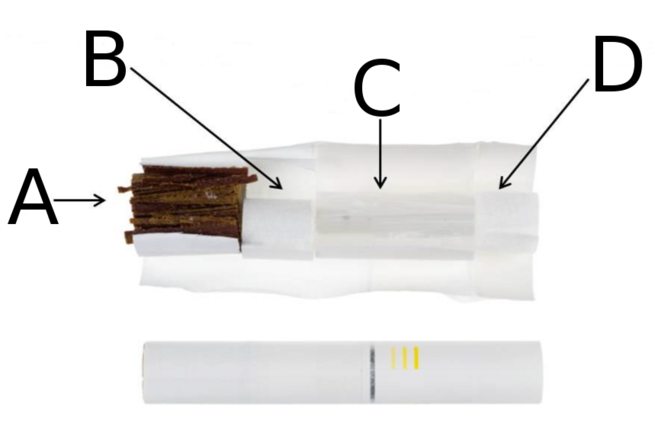
Tobacco stick; above, disassembled, below, intact.[13] A: Reconstituted tobacco film, made of dried tobacco suspension.[13] 70% tobacco, humectants (water and glycerin) to encourage aerosol formation, binding agents, and aroma agents.[13] B: Hollow acetate tube.[13] C: Polymer film filter cools the aerosol.[13] D: Soft cellulose acetate mouthpiece, which mimics the feel of a traditional cigarette.[13]
Nicotine is released from tobacco heated above 150 °C.[38] Combustible tobacco cigarettes reach about 900 °C during a puff and smoulder at about 400 °C between puffs.[5] The burning process, substances emitted and their levels vary at different temperatures.[5] Distillation, the process during which nicotine and aromas are transferred from tobacco to smoke, occurs below 300 °C; pyrolysis occurs at about 300 °C–700 °C, entails the decomposition of biopolymers, proteins, and other organic materials and generates the majority of substances emitted in smoke; and combustion occurs above 750 °C and results in the generation of carbon dioxide, carbon monoxide, and water.[5] HeatSticks are heated to a maximum of 350 °C, a temperature sufficient to enable pyrolytic decomposition of some organic materials.[5] Formation of toxic volatile organic compounds, including formaldehyde, acetaldehyde and acrolein, via dehydration and oxidation of the humectants, propylene glycol and glycerin, have been reported in e-cigarette aerosols at similar temperatures as IQOS.[5] In addition, flavoring chemicals in e-cigarettes undergo thermal degradation and contribute significantly to levels of toxic aldehydes emitted in e-cigarette aerosol.[5] Since the constituents of HeatSticks may be different from that of combustible cigarettes, including flavorants and additives, it is plausible that the IQOS aerosol may contain substances not present in tobacco smoke.[5] The IQOS HeatSticks do not generate a flame, they are charred following use.[39] Heat-not-burn tobacco products may or may not generate smoke.[10]
The heat-not-burn tobacco product consists of 3 components with different functions.[13] These include the tobacco stick with processed tobacco, a pen-like heater (holder), in which the tobacco stick is inserted, which is then heated by means of an electrically controlled heating element, and a charger (the charger), which recharges the heater after use.[13] The heat-not-burn tobacco products automatically stops the heating process after 6 minutes or 14 moves, so that pyrolysis products and pollutant release are limited in time as well as by a maximum number of puffs per stick.[13] The tobacco stick contains a compressed tobacco film as well as several filter elements.[13] The tobacco film consists of a dried tobacco suspension that has been rolled up into a paper-thin brown tobacco foil.[13] This consists of about 70% tobacco as well as humectants, binders and flavorings.[13] Water and glycerin are used as humectants to prevent drying out and to promote aerosol formation upon heating.[13] The filter elements consist of 2 independent systems: A polymer film filter that cools the aerosol, followed by a soft cellulose acetate mouthpiece filter that mimics the sensory aspects of a traditional cigarette.[13]
Heat-not-burn tobacco products are a battery-powered systems that produce nicotine-containing emissions by heating tobacco.[13] For this purpose, tobacco sticks are placed in a corresponding heater and heated to about 250–350 °C (around 500 °F.[12]).[13] This results in nicotine-containing emissions, which are inhaled via a mouthpiece with a filter segment.[13] They are hybrids between electronic and conventional cigarettes: on one hand, they are equipped with a device that heats the product, without reaching combustion, to generate aerosol (ie, a sort of "cold smoke"); on the other hand, the product used is not a liquid containing nicotine, but "real" tobacco.[37]
Heat-not-burn tobacco products heat tobacco leaves at a lower temperature than traditional cigarettes.[11] Heat-not-burn tobacco products usually heat up tobacco, rather than use liquids.[40] Another type of heat-not-burn tobacco product is the loose-leaf tobacco vaporizer that entails putting loose-leaf tobacco into a chamber, which is electrically heated using an element.[41] Some use product-specific customized cigarettes.[4] They are not e-cigarettes.[4] They can overlap with e-cigarettes such as combining an e-cigarette and a heat-not-burn tobacco product, for using tobacco or e-liquid.[15]
History
As early as the 1960s, the tobacco companies developed alternative tobacco products with the goal of supplementing the cigarette market with products.[20] The first commercial heat-not-burn product was the R. J. Reynolds Premier,[42] a smokeless cigarette launched in 1988 and described as difficult to use.[43] Many smokers disliked the taste.[44] It was shaped like a traditional cigarette, and when heated the smoldered charcoal moved past processed tobacco containing more than 50 percent glycerin to create an aerosol of nicotine.[45] It did require some combustion.[46] In 1989,[47] after spending $325 million,[48] R. J. Reynolds pulled it from the market months later after organizations recommended to the US Food and Drug Administration (FDA) to restrict it or classify it as a drug.[49]
The Premier product concept went on to be further developed and re-launched as Eclipse[47] in the mid-1990s,[50] and was available in limited distribution as of 2015.[51] Reynolds American stated that the Revo was a "repositioning" of its Eclipse.[52] R. J. Reynolds' Revo was withdrawn in 2015.[51]
Philip Morris International launched a cigarette in 1998 that was placed into an electronic heating device as Accord.[53] Also in 1998 the company launched Accord in Osaka, Japan, calling it Oasis.[54] The battery-powered product was the size of a pager.[55] The product was marketed as "low-smoke".[54] Advertisements stating reduced risk were drafted for Accord in the US, but were never released.[54] An attempt was made in 2007 by Kenneth Podraza, who was the Vice President of Research and Development at Philip Morris in the US, to get the Surgeon General of the United States to endorse it.[54] It has not been shown that the Surgeon General replied to Podraza's letter.[54] Few people started using the Accord, and most users also continued to use traditional cigarettes.[54] The Accord ceased production in 2006, eight years after it came on the market.[54]
In 2007 Philip Morris International launched Heatbar;[56] which was very similar to the Accord.[54] The Heatbar was around the size of a mobile phone and was said to heat specifically designed cigarettes rather than burning them.[57] The only benefit was to lower second-hand smoke, which lead to Heatbar being discontinued.[58] Heatbar did not obtain any significant user reception.[59] Accord and Heatbar are predecessors of Philip Morris International's heat-not-burn tobacco products.[60] Heat-not-burn tobacco products were not a commercial success, and most of them were quickly taken off the market following their debut.[12]
In recent years leading up to 2018, increased tobacco control measures have directed the tobacco industry to develop alternative tobacco products, such as heat-not-burn tobacco products.[13] There has been a global decline in tobacco consumption that, if continued, will negatively impact the tobacco industry's profits.[20] This decline led the industry to invent and market new products, such as heat-not-burn tobacco products.[20] The introduction of these heat-not-burn tobacco products may also have been a response to the growing popularity of e-cigarettes beginning around 2007 after independent companies introduced them before the major multinational tobacco companies entered the e-cigarettes market.[20] Furthermore, the global decline of cigarette consumption and decrease in adult smoking prevalence (from 24% in 2007 to 21% in 2015), combined with the success of tobacco control, including implementation of the WHO Framework Convention on Tobacco Control, may also have lead the tobacco companies to consider alternative products to protect their profits and political interests.[20] The ubiquitousness of e-cigarettes and growing dissatisfaction with they do not provide a throat-hit may present an opportunity for heat-not-burn tobacco products.[12] These products have been introduced by large tobacco companies.[21] They are developed by several tobacco companies.[61] Philip Morris International anticipates a future without traditional cigarettes, but campaigners and industry analysts call into question the probability of traditional cigarettes being dissolved, by either e-cigarettes or other products like IQOS.[62]
Products
-
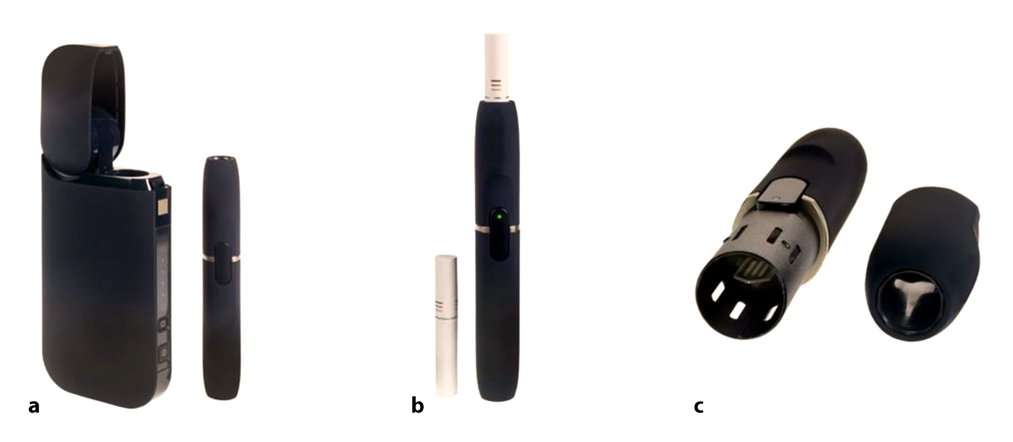
Heat-not-burn tobacco system. a) Charger (left) and holder (right), b) Tobacco stick (left) and holder with tobacco stick inserted (right), c) Disassembled holder, with heating element visible (left) and the holder's lid (right).[13]
The products use a heating-system where the tobacco is heated and aerosolized.[4] In addition to nicotine, they contain additives not derived from tobacco, and are frequently flavored.[4] The evidence indicates that the concentrations of nicotine in mainstream heat-not-burn tobacco products aerosol are less than what is found in cigarette smoke.[30] Smokers regularly reported heat-not-burn tobacco product use to be less satisfying than smoking a cigarette.[19] The heat-not-burn tobacco products that were tested provided more nicotine in the aerosol than a cigalike e-cigarette but not as much nicotine compared with a tank style e-cigarette.[19] They are designed to be similar to their combustible counterparts[52] and they provide some of the behavioral aspects of smoking.[14] These products replicate the oral inhalation and exhalation, taste, rapid systemic delivery of nicotine, hand-to-mouth feel and throat hit sensations (depending on the temperature) that are similar to smoking traditional cigarettes.[11] There are different types of heat-not-burn tobacco products in the marketplace.[25]
3T

The 3T from Vapor Tobacco Manufacturing was launched in December 2014.[63] The product employs a patented, aqueous system whereby desired components are extracted into water.[64] The liquid is mixed with glycerin and aerosolized by an electronic heating system.[64] Their organic liquids are made from organic tobacco, organic glycerin, and water.[63]
Firefly 2
The Firefly developed the Firefly 2, which heats loose-leaf plant material and concentrates and is often used to vaporize marihuana.[65]
glo
In 2016, British American Tobacco launched a battery-powered heat-not-burn product called glo in Japan.[66] It is also sold in South Korea, Switzerland, and Russia.[67] It uses a heating element with a tobacco stick,[52] which looks similar to a short cigarette.[67] In May 2017 they released i-glo in Canada.[68] The glo iFuse debuted in Romania by British American Tobacco in 2015.[52] It uses a cartridge with a tobacco stick and a flavored nicotine liquid.[52] It is a hybrid of an heat-not-burn tobacco product and an e-cigarette.[5] It consists of a heating element, a liquid tank (like e-cigarettes) and a tobacco cavity through which the e-cigarette-like aerosol passes and is infused with tobacco flavor.[5] Bonnie Herzog, a senior analyst at Wells Fargo Securities stated that the proposed acquisition of R. J. Reynolds by British American Tobacco in 2016 would let them catch up in the technology competition.[69] The data on glo is limited.[33]
IQOS

The introduction of IQOS (/ˈaɪkoʊs/ EYE-kohs[70]) was announced on June 26, 2014.[71] Some have stated IQOS stands for "I quit ordinary smoking", according to The Express Tribune.[70] IQOS was not intended to be an acronym for "I Quit Ordinary Smoking," according to chief executive officer André Calantzopoulos of Philip Morris International.[72] The company explained that the name began with a lowercase "i," then changed into "IQ" together with "OS," has no specific meaning.[72] The product is marketed by Philip Morris International under the Marlboro and Parliament brands.[73] Although it is marketed as a novel product, it is very similar to the "Accord" product released by the same company in 1998; however, the IQOS products have more nicotine, more tar, and less tobacco.[54] They are heated to a lower temperature, and the kit costs about US$40 more in 2018 dollars.[54]
Initially launched in 2014 in Nagoya, Japan and Milan, Italy, IQOS is being gradually rolled out to other countries.[74] By the end of 2016, it was available in over twenty countries, with expansion plans into several more in 2017 as manufacturing capacity increases.[75] As of May 2018, IQOS is available in over 37 countries.[5] Philip Morris International began selling IQOS and HEETS modified cigarettes in two of its stores in Seoul, Korea on May 27, 2017.[76] On June 5, 2017, other retailers in Seoul including CU and Electromart began offering them.[76] As of 2018[update] IQOS is not approved for sale in the US.[77] Philip Morris International has projected that when 30 billions units are sold, IQOS would increase profits by $700 million.[78] In December 2017, the company launched TEEPS in the Dominican Republic.[79] It is a heat-not-burn product that looks similar to a traditional cigarette.[79] In October 2018, Philip Morris International introduced a less expensive version of IQOS called IQOS 3 in Tokyo, Japan.[80]
In the UK, IQOS has been sold at an exclusive shop in London since December 2016 and later it became available online and at a few other retailers in London.[40] Outside of an IQOS retail shop in Canada, marketing included a sign with the message, 'Building a Smoke-Free Future'.[81] The packaging of IQOS is similar to iPhones and other upscale smartphones.[82] The IQOS is marketed as a "smoke-free" alternative to traditional cigarettes, and promoted as a way to lower risk from smoking.[83] Philip Morris International has been intensively promoting its IQOS product in Europe and Asia, as of 2017.[84] IQOS has over 1.4 million frequent users, according to the company, as of 2017[update].[72]
As of 2016, the company stated total investments made in the development and assessment of these products have exceeded $3 billion.[85] Phillip Morris spent €500 million on IQOS in 2016 alone.[86] Papastratos, Philip Morris International's division in Greece, intends to revamp a cigarette factory into a manufacturer of tobacco sticks for use with IQOS products.[87] Philip Morris International plans to invest in 2018 $320 million to build a second manufacturing plant in Dresden, Germany.[88] IQOS is probably going to be a major part of Philip Morris International's expansion strategy.[88]
The IQOS product consists of a charger around the size of a mobile phone and a holder that looks like a pen.[89] The product can collect personal data in regard to the smoking habits of the user.[90] Philip Morris International stated it only retrieves the data when the product is not working properly.[90] The disposable tobacco stick[91] called HeatSticks or HEETS in some places they are sold,[92] looks similar to a short cigarette.[67] The sticks contain processed tobacco and has been soaked in propylene glycol.[91] The stick is inserted into the holder which then heats it to temperatures up to 350 °C.[53] The smoke[91] and aerosol released contains nicotine, tobacco and other chemicals.[4] The amount of nicotine provided may be a little strong for light cigarette smokers.[93] The sticks are available in regular, balanced regular, menthol and mint flavor.[93] Users have reported less smell and odor on clothing.[46] Philip Morris International states that IQOS generates no smoke because the tobacco does not combust and the stick is heated rather than burned.[91] Even without fire, smoke can be produced.[91] Both IQOS and traditional cigarettes do not completely combust (pyrolysis) tobacco.[91] The emissions generated by IQOS contains substances from pyrolysis and thermogenic degradation that are identical to the constituents found in traditional tobacco cigarette smoke.[91] The emissions generated by IQOS contains the identical harmful constituents as cigarette smoke, including volatile organic compounds at comparable levels to cigarette smoke, polycyclic aromatic hydrocarbons at vast various ranges, and carbon monoxide.[1] All of these substances, on the basis of rigorous research of cigarette smoke, are known to cause significant harms to health.[1] A 2017 review found "little research on what substances are released after the device heats the tobacco-based paste. The physical effects on users are also not yet known."[84] Carlos Jiménez, director of research on smoking at the Spanish Society of Pneumonology and Thoracic Surgery stated in 2017 that the IQOS product is still harmful.[94] IQOS is likely less toxic than traditional cigarettes.[23]
A 2017 independent study of the IQOS states that "Dancing around the definition of smoke to avoid indoor-smoking bans is unethical" and called for more independent research, stating "Smokers and non-smokers need accurate information about toxic compounds released in IQOS smoke. This information should come from sources independent of the tobacco industry".[91] In 2017, according to two editors of the journal JAMA Internal Medicine, after publication of a research letter describing harmful chemicals in heat-not-burn tobacco products, people from Philip Morris International contacted the institutions where the researchers worked and questioned the methods used in the study; the editors described this as a form of "pressure to suppress discourse that could harm commercial interests".[95] Philip Morris International asked the University of Lausanne to retract the study.[96] A spokeswoman for the University of Lausanne stated in an email that following the release of their study, the heads of the University of Bern, Lausanne University Hospital, and University of Lausanne where the authors worked received letters from Phillip Morris International, criticizing the authors for using a flawed methodology in the study.[97] When contacted by a journalist from The Washington Post, the researchers declined to comment.[97] Philip Morris International had published an academic counter-argument on the Internet.[97] Philip Morris International told CBS This Morning in January 2018 that the IQOS product produces no smoke.[98] Reto Auer, who directed the 2017 study, said in an interview prior to the US FDA two-day hearing, "We disagree with the claim that it's smokeless."[99] The FDA stated in January 2018 that "There are significant analytical issues in the Auer et al. study, such as lack of testing reference samples, low number of replicates, lack of selectivity on some analytical methods."[100] In December 2017, Reuters published documents and testimonies of former employees detailing irregularities in the clinical trials conducted by Philip Morris International for the approval of the IQOS product by the US FDA.[101]
On December 5, 2016,[102] Philip Morris International submitted a multi-million page application[86] to the US FDA for IQOS to be authorized as a modified risk tobacco product.[102] Then in late March of 2017, Philip Morris International submitted to the US FDA for a premarket tobacco product application regarding its iQOS product.[103] In May 2017, Philip Morris International had received notice that the US FDA had started a lengthy scientific review process for the IQOS product.[103] On 24 May 2017, the US FDA had published papers acknowledging Philip Morris International's modified risk tobacco product application regarding the IQOS product.[104]
The advisory panel appointed by the US FDA reviewed Philip Morris International’s application in January 2018.[99] Matthew Myers, representing the Campaign for Tobacco-Free Kids, told the US FDA advisory panel that "It is high-tech. It is sleek. It is designed in exactly the way that would appeal to young people."[99] Jeff Fortenbacher, CEO of Access Health, stated that "Patients who smoke clearly need more tools to help them quit."[99] The advisory panel voted in favor of the claim that IQOS reduced users' exposure to harmful chemicals.[99] The US FDA advisory panel stated that Philip Morris International did not demonstrate that the product reduces the risks of diseases associated with tobacco use.[105] Philip Morris International's claim that "switching completely to iQOS presents less risk of harm than continuing to smoke cigarettes" did not gain support by the US FDA advisory panel.[99] The panel also "expressed concerns about the lack of data" on risk relative to traditional cigarettes.[106] The US FDA reviewed Phillip Morris International's data, independent studies, including a 2017 study on IQOS and a posted comment on Phillip Morris International's website about the 2017 study, a December 8, 2017 amendment to the application by Phillip Morris International on the same topic, and the US FDA's own laboratory testing data.[100] The FDA intends to continue to review Philip Morris International's research.[107]
Philip Morris International intends to convert its customers in Japan to using heat-not-burn products.[108] The IQOS products are sold as an alternative to regular cigarettes.[95] Philip Morris International conducted studies using their IQOS product.[109] The participants in the studies who were given the IQOS product were not likely to quit using traditional cigarettes.[109] In 2016 PMI acknowledged that the IQOS product is probably as addictive as tobacco smoking.[27] IQOS is sold with a warning that states the best option is to avoid tobacco use altogether.[110]
iSmoke OneHitter
iSmoke OneHitter by iSmoke was launched in 2015.[111] It can be used as a loose-leaf tobacco vaporizer.[112] It has a chamber that can be filled with up to 800-milligrams of tobacco.[112]
IUOC
IUOC, marketed by Shenzhen Yukan Technology Co., Limited from China, can be used with any brand of cigarettes.[113] Users insert the entire cigarette into the product.[113] It does not use a tobacco stick.[113] The temperature reaches up to 395 °C when in use.[113] IUOC can smoke around 10 cigarettes per charge.[113]
lil
Korea Tobacco & Ginseng Corporation announced on June 8, 2017 that they will launch a heat-not-burn tobacco product in September 2017.[114] The heat-not-burn tobacco product named lil formally launched in November 2017.[115] The battery-operated product employs heat to the tobacco leaves.[115]
Pax 2
PAX Labs, formerly known as Ploom,[116] sells PAX vaporizers.[117] In 2010 they launched Ploom, a butane-powered product used for the heating tobacco or botanical products.[118] Later models replaced butane heating with an electric system.[119] After its initial partnership with Japan Tobacco was abandoned, the company became known as Pax Labs.[120] The Pax 2 uses loose-leaf tobacco.[52] The surface of the Pax 2 remains cool, while the oven heats to temperatures up to 455 °F.[121] It has four temperature options.[121]
Ploom Tech
In January 2016, Japan Tobacco released Ploom Tech.[122] Japan Tobacco's Ploom has been withdrawn from the US.[52] The Ploom brand, however, remained with Japan Tobacco and the product itself has been replaced with a different product called Ploom Tech, in which an aerosol passes through a capsule of granulated tobacco leaves.[123] In January 2019, they introduced Ploom TECH+ and Ploom S in Tokyo, Japan.[124]
Sales are being expanded throughout Japan in 2017.[125] They intend to spend $500 million to increase their heated tobacco manufacturing capacity by late 2018.[126] Studies on Japan Tobacco International's Ploom product has not been found.[33]
Pulze
Imperial Brands is working on a heat-not-burn tobacco product named Pulze.[127]
V2 Pro
V2 originally released their vaporizer line named V2 Pro in July 2014.[128] The initial product was named Series 3.[128] Series 3 comes with 3 cartridges including a loose-leaf cartridge, which heats the material by conduction.[129] It comes with a battery and USB changer, among other things.[129] Pro Series 3X also by V2 can be used with dry material.[130] It has three different air flow options that can be adjusted with a slight turn of the mouthpiece.[130] Series 7 comes with a loose-leaf cartridge, among other things.[131] Series 7 lets the user change the temperature by using a single button.[128]
Comparison to traditional cigarettes
| Analyzed Substance | IQOS product; Amount, Mean (SD) | Duplicate tests for given assay | Traditional cigarettes; Amount, Mean (SD) | Duplicate tests for given assay | Percentage (%) of the substance in each IQOS compared to traditional cigarettes |
|---|---|---|---|---|---|
| Volatile organic substances, μg per cigarette: 1 | |||||
| Acetaldehyde | 133 (35) | 5 | 610: 2 | 1 | 22 |
| Acetone | 12.0 (12.9) | 5 | 95.5 (13.5) | 2 | 13 |
| Acroleine | 0.9 (0.6) | 2 | 1.1 | 1 | 82 |
| Benzaldehyde | 1.2 (1.4) | 5 | 2.4 (2.6) | 2 | 50 |
| Crotonaldehyde | 0.7 (0.9) | 5 | 17.4 | 1 | 4 |
| Formaldehyde | 3.2 (2.7) | 5 | 4.3 (0.4) | 2 | 74 |
| Isovaleraldehyde | 3.5 (3.1) | 5 | 8.5 (10.8) | 2 | 41 |
| Propionaldehyde | 7.8 (4.3) | 5 | 29.6 (36.6) | 2 | 26 |
| Polycyclic aromatic hydrocarbons, ng per cigarette: 3 | |||||
| Naphthalene | 1.6 (0.5) | 4 | 1105 (269) | 7 | 0.1 |
| Acenaphthylene | 1.9 (0.6) | 4 | 235 (39) | 7 | 0.8 |
| Acenaphthene | 145 (54) | 4 | 49 (9) | 7 | 295 |
| Fluorene | 1.5 (0.6) | 4 | 371 (56) | 7 | 0.4 |
| Anthracene | 0.3 (0.1) | 4 | 130 (18) | 7 | 0.2 |
| Phenanthrene | 2.0 (0.2) | 4 | 292 (44) | 7 | 0.7 |
| Fluoranthene | 7.3 (1.1) | 4 | 123 (18) | 7 | 6 |
| Pyrene | 6.4 (1.1) | 4 | 89 (15) | 7 | 7 |
| Benz[a]anthracene | 1.8 (0.4) | 4 | 33 (4.2) | 7 | 6 |
| Chrysene | 1.5 (0.3) | 4 | 48 (6.2) | 7 | 3 |
| Benzo[b]fluoranthene | 0.5 (0.2) | 4 | 24 (2.9) | 7 | 2 |
| Benzo[k]fluoranthene | 0.4 (0.2) | 4 | 4.3 (2.8) | 7 | 9 |
| Benzo[a]pyrene | 0.8 (0.1) | 4 | 20 (2.9) | 7 | 4 |
| Indeno[1,2,3-cd]pyrene | ND | 4 | NA | NA | NA |
| Benzo[ghi]perylene | ND | 4 | NA | NA | NA |
| Dibenzo[a,h]anthracene | ND | 4 | NA | NA | NA |
| Inorganics, ppm in the mainstream smoked: 4 | |||||
| Carbon dioxide | 3057 (532) | 5 | >9000 | 3 | NA |
| Carbon monoxide | 328 (76) | 5 | >2000 | 3 | NA |
| Nitric oxide | 5.5 (1.5) | 5 | 89.4 (71.6) | 3 | 6 |
| Other evaluations | |||||
| Nicotine, μg per cigarette: 1 | 301 (213) | 4 | 361 | 1 | 84 |
| Temperature, °C | 330 (10) | 2 | 684 (197) | 1 | NA |
| Number of puffs | 12.6 (2.4) | 32 | 13.3 (3.1) | 6 | NA |
Abbreviations: NA, not analyzed; ND, not detected.[91] : 1 The techniques applied were presented earlier in Varlet et al([132]) to analyze volatile organic compounds and nicotine.[91] : 2 Due to there being one duplicate test, no SD can be determined.[91] : 3 The values presented were illustrated from Vu et al([133]) for the ISO smoking regimen and for an average of the 35 highest selling US traditional cigarette brands.[91] : 4 Carbon dioxide was assessed with a Testo 535 (Testo), and carbon monoxide and nitric oxide were assessed with a Pac 7000 that identified carbon monoxide (Draeger).[91] The apparatus calculated the smoke whenever generated from the syringe pump.[91]
∗A 2017 analysis comparing IQOS to popular US sold traditional cigarettes.[91]
Contents of selected analytes in the mainstream smoke of a heat-not-burn tobacco product and traditional cigarettes.[13] The highest and lowest values given by Mallock et al. in 2 different types of tobacco sticks and Counts et al. were found in traditional cigarettes.[13] Column 5 shows the reduction of the analytes in the mainstream smoke of the heat-not-burn tobacco product compared to traditional cigarettes in % shown.[13]
Tobacco stick, d. h. for heat-not-burn tobacco products: a tobacco stick; for traditional cigarette: a cigarette[13] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Prevalence
Heat-not-burn tobacco products are being introduced in markets around the world, as of 2017.[134] Since mid-2017, heat-not-burn tobacco products were sold in 27 countries.[135] As of 2017, the market for such products is anticipated to expand by 60% over the next 10 to 15 years.[9] They are globally not as popular as the e-cigarette.[11] As of 2018, the IQOS is the most popular product.[11]
As of April 2018, the industry was rapidly introducing new heat-not-burn tobacco products.[20] As of January 2018, British American Tobacco's iFuse is available in Romania, Japan, Switzerland, Canada, South Korea, and Russia.[20] As of January 2018, Japan Tobacco International's Ploom TECH's is available in Japan and Switzerland.[20] As of January 2018, KT&G Corp.'s lil is available in South Korea.[20] As of January 2018, Philip Morris International's IQOS is available in Canada, Guatemala, Colombia, Czech Republic, Denmark, France, Germany, Greece, Israel, Italy, Kazakhstan, Lithuania, Monaco, Netherlands, Poland, Portugal, Romania, Russia, Serbia, Slovak Republic, Slovenia, Spain, Switzerland, Ukraine, UK, South Africa, South Korea, Japan, and New Zealand.[20] Heat-not-burn tobacco products were first sold in Japan.[136] Several heat-not-burn tobacco brands are marketed in Japan since 2014.[134]
Tobacco industry leaders have predicted heat-not-burn tobacco products are poised to further displace traditional cigarette smoking and, by extension, tobacco control strategies typically framed around cigarettes.[134] Yet, little is known about the popularity of these products.[134]
Heat-not-burn searches originating in Japan have experienced tremendous growth.[134] Since the introduction of Philip Morris International's IQOS brand in select Japanese cities in November 2014, searches for heat-not-burn products have increased substantially.[134] Average monthly searches rose 1,426% (95%CI: 746–3,574) between the first (2015) and second (2016) complete years heat-not-burn tobacco was marketed.[134] Queries for heat-not-burn products continued to grow an additional 100% (95%CI: 60–173) between the products second (2016) and third years on the market (Jan-Sep 2017).[134] In practical terms, there are now between 5.9 and 7.5 million heat-not-burn related Google searches in Japan each month based on the latest search estimates for September 2017.[134] Moreover, forecasts relying on the historical trend suggest heat-not-burn searches will increase an additional 32% (95%CI: -4 to 79) during 2018, compared to current estimates for 2017 (January-September), with further growth expected.[134]
Demand for heat-not-burn tobacco products presents a host of tobacco control challenges similar to e-cigarettes and new challenges specific to these products.[134] Heat-not-burn tobacco products have been advertised as reduced-risk tobacco products in their Japanese test market, and these marketing messages will undoubtedly contaminate other markets even where such messaging is banned.[134] Tobacco control advocates will have to develop strategies to both discover and then disseminate messages about the health risks associated with these products.[134] Further, tobacco control advocates will have to consider making adjustments to existing tobacco control policies so that they apply to heat-not-burn tobacco products.[134] For example, policy makers want to extend existing indoor smoking bans to include the emissions from heat-not-burn tobacco products, whether to stigmatize their use or protect bystanders from potentially hazardous emissions.[134]
Marketing
The term "heat-not-burn" refers to tobacco heated (at ~350 °C) by an electrically-powered element or carbon, not combusted (at ~800 °C).[11] Terms used in marketing of cigarette-like products that "heat rather than burn" are referring to the product as "reduced risk" and "innovative."[65] Marketing slogans like "heat-not-burn" cannot be a substitute for science.[91] Heat-not-burn tobacco products are not typically marketed as a harmless substitute to smoking.[26]
The tobacco companies are using a series of claims in the marketing of heat-not-burn tobacco products.[20] Both in websites and statements to the media and investors, heat-not-burn tobacco products are presented as less harmful but not risk-free.[20] In a few instances, marketing materials claim that heat-not-burn tobacco products are potentially helpful to smokers who want to quit.[20] Some media accounts of product launches state that heat-not-burn tobacco products reduce the levels of harmful tobacco components by 90%–95% compared with traditional cigarettes, while others emphasise the lack of odor or visible emissions as part of marketing campaigns.[20] As of April 2018, there is no evidence to confirm this claimed 90%–95% lower level of harm.[20] Other marketing claims highlight that these products produce no smoke, that is, are smoke-free.[20] Implied in these claims, in advertisements and stores globally, is that smokers should switch from traditional cigarettes to these new, allegedly less harmful, products.[20] The introduction of the latest generation of heat-not-burn tobacco products appears to be the latest chapter in the decades-old tobacco industry strategy of working to create partnerships with governments and health advocates, presenting these alleged 'harm reduction' products as an option to address the tobacco epidemic.[20]
The tobacco industry's use of the 'harm reduction' framework also serves to fracture the tobacco control movement, leaving it without a unified voice to communicate with the public, the media and with policy makers on the strategies to advance tobacco control.[20] The concept of harm reduction has traditionally been embraced in several public health fields such as clean needles for injectable drug use and has been explored by some tobacco control experts in the past, with enthusiasm for the possibility of harm reduction growing with the widespread availability of e-cigarettes in certain markets.[20] The tobacco industry frames harm reduction as a common ground with health advocates and a possible entry point to influence legislation and regulation of tobacco products.[20]
The tobacco companies use heat-not-burn tobacco products as part of their broader political and public relations activities to position them as 'partners' to address the tobacco epidemic rather than as the vectors that are causing it.[20] This is a similar strategy previously used by the tobacco industry to promote itself as a partner of public health in reducing the harms of tobacco, while obfuscating the scientific evidence pointing that harm reduction is achieved through tobacco control policies that decrease consumption.[20]
Regulation
Current smoking bans may not have been extended to include such products.[22] In the majority of the countries in which they have been sold, heat-not-burn tobacco products have been taxed at a lower rate than traditional cigarettes.[137]
"There is concern that heat-not-burn tobacco will skirt local ordinances that prevent smoking in public areas," Mitchell H. Katz, director of the Los Angeles County Health Agency, wrote in 2017.[22] Action on Smoking and Health stated in 2016 that "unless and until independent evidence shows that IQOS and similar products are substantially less harmful than smoking then these products should be regulated in the same way as other tobacco products."[27] Tobacco control activist Stanton Glantz stated that the US FDA should halt new tobacco products until tobacco companies stop selling traditional cigarettes.[138] It is recommended that indoor-smoking bans for traditional cigarettes be extended to heat-not-burn tobacco products.[91] It is recommended that marketing of these products, and claims being made about them, should be regulated.[20]
In the United States, these products fall under the jurisdiction of the Food and Drug Administration as amended by the Family Smoking Prevention and Tobacco Control Act of 2016.[41]
Advertisement for the IQOS product itself is not regulated under the European Union Tobacco Products Directive.[52] Advertising for IQOS' tobacco stick may fall under the European Union Tobacco Products Directive.[52] The UK government has been looking into creating a separate category for taxing heat-not-burn tobacco products.[40]
Due to the alleged belief in heat-not-burn tobacco harm reduction in Italy, these products are exempted from the fiscal regimes of tobacco products.[37] Heat-not-burn tobacco products enjoy the same tax reduction as e-cigarettes, which is half that of traditional cigarettes.[37] Moreover, the enforcement of various tobacco control regulations is only minimally adopted for heated tobacco products in Italy.[37] First of all, health warnings are required to cover only 30% of the heat-not-burn tobacco product packaging (instead of 65% for traditional cigarettes), without pictorial images.[37] Second, comprehensive smoke-free regulations prohibiting smoking in all public places and workplaces do not apply to heat-not-burn tobacco products.[37] Finally, advertising and promotions are not banned for these new products.[37] This is evident by the presence in several strategic Italian cities of the "IQOS embassy" and "IQOS boutique", which are fancy concept stores where IQOS is promoted as a status symbol and people can try it for free.[37] Therefore, the most recognized tobacco control policies (ie, price/tax increase, smoking bans, advertising bans, and health warnings) have been compromised for heat-not-burn tobacco products in Italy.[37]
Heat-not-burn tobacco products are not restricted for sale in Israel by the Ministry of Health.[139] Justice Ministry in Israel agreed with the view of three voluntary organizations that the IQOS is a tobacco product, and the product should be regulated in the same manner as tobacco products.[140] In Israel IQOS are now taxed at the same rate as traditional cigarettes.[141]
Ploom, IQOS, and glo fall under the Tobacco Business Act as tobacco products in Japan because they consist of tobacco leaf.[35] Ploom and IQOS are governed by the Tobacco Industries Act regulations as tobacco products in Japan.[142] The Liberal Democratic Party will deliberate over increasing the tax rate for heat-not-burn tobacco products in April 2018.[143]
Electronic tobacco products using dry material are regulated as e-cigarettes in South Korea by the Ministry of Health and Welfare.[144] Korea regulates e-cigarettes differently than traditional cigarettes for tax reasons.[145] As a result, IQOS are taxed at a decreased rate, compared to the 75% incurred on normal cigarettes.[145] Emerging tobacco products are banned in Singapore by the Ministry of Health.[146]
After IQOS launched a marketing campaign in New Zealand in December 2016, the Ministry of Health stated in 2017 that the refill sticks are not legal for sale in New Zealand under the Smoke-free Environments Act 1990.[147] A representative for the company in New Zealand stated that IQOS product complies with the Smoke-Free Environments Act.[148] Three meetings between Ministry of Health officials and people from the tobacco industry were held from May 30, 2017 through June 2, 2017 to "discuss regulation of new tobacco and nicotine-delivery products".[149] Later on, in August 2017, the government stated they would initiate a review process before products are sold for heat-not-burn tobacco products such as IQOS.[149] In 2018, Philip Morris International and the Ministry of Health were in a court over the legality of selling IQOS in New Zealand.[150] A New Zealand court decided in March 2018 that the HEETs sticks used in the IQOS product are legal to sell in New Zealand.[151] Individuals can import heat-not-burn tobacco products to New Zealand for personal use.[152] As of 2016[update], 19 countries have permitted the sale of IQOS.[91]
Notes
- ^ A heat-not-burn tobacco product (HNB),[1] is also variously known as heat-not-burn product,[2] heat-not-burn cigarette (HC),[3] heated tobacco product (HTB),[4] electronic heated tobacco product,[5] electronically-heated cigarette smoking system (EHCSS),[6] tobacco heating system,[7] smokeless tobacco stick,[8] or a T-vapor.[9]
Bibliography
- McNeill, A; Brose, LS; Calder, R; Bauld, L; Robson, D (February 2018). "Evidence review of e-cigarettes and heated tobacco products 2018" (PDF). UK: Public Health England. pp. 1–243.
- "Regulatory Impact Statement: Regulation of smokeless tobacco and nicotine-delivery products" (PDF). Ministry of Health (New Zealand). 2017. pp. 1–52.
- "Further development of the partial guidelines for implementation of Articles 9 and 10 of the WHO FCTC" (PDF). World Health Organization. 12 July 2016. pp. 1–11.
References
- ^ a b c d e f g Jenssen, Brian P.; Walley, Susan C.; McGrath-Morrow, Sharon A. (2017). "Heat-not-Burn Tobacco Products: Tobacco Industry Claims No Substitute for Science". Pediatrics. 141 (1): e20172383. doi:10.1542/peds.2017-2383. ISSN 0031-4005. PMID 29233936.
- ^ Leigh, Noel J; Tran, Phillip L; O’Connor, Richard J; Goniewicz, Maciej Lukasz (2018). "Cytotoxic effects of heated tobacco products (HTP) on human bronchial epithelial cells". Tobacco Control. 27 (Suppl 1): s26–s29. doi:10.1136/tobaccocontrol-2018-054317. ISSN 0964-4563. PMC 6252481. PMID 30185530.
- ^ Kamada, Takahiro; Yamashita, Yosuke; Tomioka, Hiromi (2016). "Acute eosinophilic pneumonia following heat-not-burn cigarette smoking". Respirology Case Reports. 4 (6): e00190. doi:10.1002/rcr2.190. ISSN 2051-3380. PMC 5167280. PMID 28031826.
- ^ a b c d e f g h i j k l m n o p "Heated tobacco products (HTPs) information sheet". World Health Organization. 2018.
- ^ a b c d e f g h i j k St.Helen, Gideon; Jacob III, Peyton; Nardone, Natalie; Benowitz, Neal L (2018). "IQOS: examination of Philip Morris International's claim of reduced exposure". Tobacco Control. 27 (Suppl 1): s30–s36. doi:10.1136/tobaccocontrol-2018-054321. ISSN 0964-4563. PMC 6252487. PMID 30158205.
 This article incorporates text by Gideon St.Helen, Peyton Jacob III, Natalie Nardone, and Neal L Benowitz available under the CC BY 4.0 license.
This article incorporates text by Gideon St.Helen, Peyton Jacob III, Natalie Nardone, and Neal L Benowitz available under the CC BY 4.0 license.
- ^ a b Lindson-Hawley, Nicola; Hartmann-Boyce, Jamie; Fanshawe, Thomas R; Begh, Rachna; Farley, Amanda; Lancaster, Tim (2016). "Interventions to reduce harm from continued tobacco use". Cochrane Database of Systematic Reviews. 10: CD005231. doi:10.1002/14651858.CD005231.pub3. ISSN 1465-1858. PMID 27734465.
- ^ McNeill 2018, p. 30.
- ^ "Philip Morris' Smokeless Tobacco Stick Shouldn't Be Marketed As Safer Than Cigarettes, FDA Panel Says". Kaiser Health News. 26 January 2018.
- ^ a b Unger, Michael; Unger, Darian W. (2018). "E-cigarettes/electronic nicotine delivery systems: a word of caution on health and new product development". Journal of Thoracic Disease. 10 (S22): S2588–S2592. doi:10.21037/jtd.2018.07.99. ISSN 2072-1439. PMC 6178300. PMID 30345095.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Katz, Mitchell H. (July 2017). "No Smoke—Just Cancer-Causing Chemicals". JAMA Internal Medicine. 177 (7): 1052. doi:10.1001/jamainternmed.2017.1425. ISSN 2168-6106. PMID 28531245.
- ^ a b c d e f g h i j k l m n o p q r s t u v Li, Gerard; Saad, Sonia; Oliver, Brian; Chen, Hui (2018). "Heat or Burn? Impacts of Intrauterine Tobacco Smoke and E-Cigarette Vapor Exposure on the Offspring's Health Outcome". Toxics. 6 (3): 43. doi:10.3390/toxics6030043. ISSN 2305-6304. PMC 6160993. PMID 30071638.
{{cite journal}}: CS1 maint: unflagged free DOI (link) This article incorporates text by Gerard Li, Sonia Saad, Brian G. Oliver, and Hui Chen available under the CC BY 4.0 license.
This article incorporates text by Gerard Li, Sonia Saad, Brian G. Oliver, and Hui Chen available under the CC BY 4.0 license.
- ^ a b c d e Caputi, TL (24 August 2016). "Heat-not-burn tobacco products are about to reach their boiling point". Tobacco Control. 26 (5): 609–610. doi:10.1136/tobaccocontrol-2016-053264. PMID 27558827.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Pieper, Elke; Mallock, Nadja; Henkler-Stephani, Frank; Luch, Andreas (2018). "Tabakerhitzer als neues Produkt der Tabakindustrie: Gesundheitliche Risiken" ["Heat not burn" tobacco devices as new tobacco industry products: health risks]. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (in German). 61 (11): 1422–1428. doi:10.1007/s00103-018-2823-y. ISSN 1436-9990. PMID 30284624.
 This article incorporates text by Elke Pieper, Nadja Mallock, Frank Henkler-Stephani, and Andreas Luch available under the CC BY 4.0 license.
This article incorporates text by Elke Pieper, Nadja Mallock, Frank Henkler-Stephani, and Andreas Luch available under the CC BY 4.0 license.
- ^ a b Bentley, Guy (15 March 2017). "Heat-Not-Burn Tobacco: The Next Wave Of A Harm-Reduction Revolution". Forbes.
- ^ a b c d MHNZ 2017, p. 4.
- ^ a b c WHO 2016, p. 6.
- ^ a b c McNeill 2018, p. 220.
- ^ a b c d e f g h i j "Toxicological evaluation of novel heat-not-burn tobacco products – non-technical summary" (PDF). Committee on Toxicity. 11 December 2017. pp. 1–4.
- ^ a b c d e McNeill 2018, p. 23.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab Bialous, Stella A; Glantz, Stanton A (2018). "Heated tobacco products: another tobacco industry global strategy to slow progress in tobacco control". Tobacco Control. 27 (Suppl 1): s111–s117. doi:10.1136/tobaccocontrol-2018-054340. ISSN 0964-4563. PMC 6202178. PMID 30209207.
 This article incorporates text by Stella A Bialous and Stanton A Glantz available under the CC BY 4.0 license.
This article incorporates text by Stella A Bialous and Stanton A Glantz available under the CC BY 4.0 license.
- ^ a b Chin, Neo Chai (2 March 2017). "Heated tobacco products just as bad as cigarettes: Amy Khor". Today (Singapore newspaper). Mediacorp.
- ^ a b c Rapaport, Lisa (26 May 2017). "'Heat-not-burn' cigarettes still release cancer-causing chemicals". Reuters.
- ^ a b "Addictive nicotine and harmful substances also present in heated tobacco". Netherlands National Institute for Public Health and the Environment. 15 May 2018.
- ^ a b c "Alternatieve tabaksproducten: harm reduction?" [Alternative tobacco products: harm reduction?] (PDF). Netherlands National Institute for Public Health and the Environment. 2016. p. 5.
- ^ a b c d McNeill 2018, p. 219.
- ^ a b c d e f g h Kotz, Daniel; Kastaun, Sabrina (2018). "E-Zigaretten und Tabakerhitzer: repräsentative Daten zu Konsumverhalten und assoziierten Faktoren in der deutschen Bevölkerung (die DEBRA-Studie)" [E-cigarettes and heat-not-burn products: representative data on consumer behaviour and associated factors in the German population (the DEBRA study)]. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (in German). 61 (11): 1407–1414. doi:10.1007/s00103-018-2827-7. ISSN 1436-9990. PMID 30284626.
- ^ a b c "ASH reaction to new Philip Morris IQOS 'heat not burn' product". ASH UK. 30 November 2016.
- ^ Kaur, Gurjot; Muthumalage, Thivanka; Rahman, Irfan (2018). "Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products". Toxicology Letters. 288: 143–155. doi:10.1016/j.toxlet.2018.02.025. ISSN 0378-4274. PMID 29481849.
- ^ a b c Kaunelienė, Violeta; Meišutovič-Akhtarieva, Marija; Martuzevičius, Dainius (2018). "A review of the impacts of tobacco heating system on indoor air quality versus conventional pollution sources". Chemosphere. 206: 568–578. Bibcode:2018Chmsp.206..568K. doi:10.1016/j.chemosphere.2018.05.039. ISSN 0045-6535. PMID 29778082.
- ^ a b McNeill 2018, p. 208.
- ^ Dautzenberg, B.; Dautzenberg, M.-D. (2018). "Le tabac chauffé : revue systématique de la littérature" [Systematic analysis of the scientific literature on heated tobacco]. Revue des Maladies Respiratoires (in French). doi:10.1016/j.rmr.2018.10.010. ISSN 0761-8425. PMID 30429092.
- ^ a b McNeill 2018, p. 210.
- ^ a b c MHNZ 2017, p. 5.
- ^ a b c "Statement on the toxicological evaluation of novel heat-not-burn tobacco product" (PDF). Committee on Toxicity. 11 December 2017. pp. 1–10.
- ^ a b Tabuchi, Takahiro; Gallus, Silvano; Shinozaki, Tomohiro; Nakaya, Tomoki; Kunugita, Naoki; Colwell, Brian (2018). "Heat-not-burn tobacco product use in Japan: its prevalence, predictors and perceived symptoms from exposure to secondhand heat-not-burn tobacco aerosol". Tobacco Control. 27 (e1): e25–e33. doi:10.1136/tobaccocontrol-2017-053947. ISSN 0964-4563. PMC 6073918. PMID 29248896.
- ^ Marynak, Kristy L.; Wang, Teresa W.; King, Brian A.; Agaku, Israel T.; Reimels, Elizabeth A.; Graffunder, Corinne M. (2018). "Awareness and Ever Use of "Heat-Not-Burn" Tobacco Products Among U.S. Adults, 2017". American Journal of Preventive Medicine. 55 (4): 551–554. doi:10.1016/j.amepre.2018.04.031. ISSN 0749-3797. PMID 30033025.
- ^ a b c d e f g h i j k l Liu, Xiaoqiu; Lugo, Alessandra; Spizzichino, Lorenzo; Tabuchi, Takahiro; Gorini, Giuseppe; Gallus, Silvano (2018). "Heat-Not-Burn Tobacco Products Are Getting Hot in Italy". Journal of Epidemiology. 28 (5): 274–275. doi:10.2188/jea.JE20180040. ISSN 0917-5040. PMC 5911679. PMID 29657258.
 This article incorporates text by Xiaoqiu Liu, Alessandra Lugo, Lorenzo Spizzichino, Takahiro Tabuchi, Giuseppe Gorini, and Silvano Gallus available under the CC BY 4.0 license.
This article incorporates text by Xiaoqiu Liu, Alessandra Lugo, Lorenzo Spizzichino, Takahiro Tabuchi, Giuseppe Gorini, and Silvano Gallus available under the CC BY 4.0 license.
- ^ Forster, Mark; Liu, Chuan; Duke, Martin G; McAdam, Kevin G; Proctor, Christopher J (2015). "An experimental method to study emissions from heated tobacco between 100-200°C". Chemistry Central Journal. 9 (1): 20. doi:10.1186/s13065-015-0096-1. ISSN 1752-153X. PMC 4418098. PMID 25941536.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Davis, Barbara; Williams, Monique; Talbot, Prue (20 February 2018). "iQOS: evidence of pyrolysis and release of a toxicant from plastic". Tobacco Control. 28 (1): tobaccocontrol–2017–054104. doi:10.1136/tobaccocontrol-2017-054104 (inactive 2019-01-18). ISSN 0964-4563. PMID 29535257.
{{cite journal}}: CS1 maint: DOI inactive as of January 2019 (link) - ^ a b c McNeill 2018, p. 201.
- ^ a b Lopez, Alexa A.; Hiler, Marzena; Maloney, Sarah; Eissenberg, Thomas; Breland, Alison B. (2016). "Expanding clinical laboratory tobacco product evaluation methods to loose-leaf tobacco vaporizers". Drug and Alcohol Dependence. 169: 33–40. doi:10.1016/j.drugalcdep.2016.10.005. ISSN 0376-8716. PMC 5140724. PMID 27768968.
- ^ Mcgill, Douglas C (19 November 1988). "'Smokeless' Cigarette's Hapless Start". The New York Times. ISSN 0362-4331.
- ^ Haig, Matt (2003). Brand Failures: The Truth about the 100 Biggest Branding Mistakes of All Time. Kogan Page Publishers. ISBN 978-0-7494-4433-4.
- ^ Parker-Pope, Tara (10 February 2001). ""Safer" Cigarettes: A History". PBS.
- ^ Hilts, Philip J. (27 November 1994). "Little Smoke, Little Tar, but Full Dose of Nicotine". The New York Times. ISSN 0362-4331.
- ^ a b O'Connell, Dominic (30 November 2016). "Philip Morris could stop making conventional cigarettes". BBC News.
- ^ a b Anderson, S J; Ling, P M (2008). ""And they told two friends...and so on": RJ Reynolds' viral marketing of Eclipse and its potential to mislead the public". Tobacco Control. 17 (4): 222–229. doi:10.1136/tc.2007.024273. ISSN 0964-4563. PMC 2845302. PMID 18332064.
- ^ Haig, Matt (2005). Brand Failures: The Truth about the 100 Biggest Branding Mistakes of All Time. Kogan Page Publishers. pp. 51–. ISBN 978-0-7494-4433-4.
- ^ Fisher, Daniel (29 May 2014). "Is This The Cigarette Of The Future, And Will The FDA Let You Buy It?". Forbes.
- ^ "New heat-not-burn brand from RAI". Tobacco Journal International. 5 January 2015.
- ^ a b Craver, Richard (28 July 2015). "Reynolds ends Revo test market in Wisconsin". Winston-Salem Journal.
- ^ a b c d e f g h i Harlay, Jérôme (9 November 2016). "What you need to know about Heat-not-Burn (HNB) cigarettes". VapingPost.
- ^ a b Rossel, Stefanie (1 June 2016). "All eyes on iQOS". Tobacco Reporter.
- ^ a b c d e f g h i j Elias, Jesse; Dutra, Lauren M; St. Helen, Gideon; Ling, Pamela M (2018). "Revolution or redux? Assessing IQOS through a precursor product". Tobacco Control. 27 (Suppl 1): s102–s110. doi:10.1136/tobaccocontrol-2018-054327. ISSN 0964-4563. PMC 6238084. PMID 30305324.
- ^ Pollack, Juddan (27 October 1997). "Philip Morris tries smokeless Accord: tobacco marketer, cautious about brand, doing 'consumer research'". Ad Age.
- ^ "Anti-smoking body attacks smokeless cigarette device". Tobacco Journal International. 11 December 2007.
- ^ Houston, Cameron (27 June 2007). "Revealed: tobacco giant's secret new weapon in the age of smoking bans". The Age.
- ^ Cooper, Ted (1 February 2014). "Why Philip Morris International's New Heated Products Will Do Better Than Its Last Attempt". The Motley Fool.
- ^ Lubin, Gus (25 June 2012). "Philip Morris Is Releasing A Bunch Of Crazy New Cigarettes". Business Insider.
- ^ MacGuill, Shane (23 January 2014). "Has Philip Morris Learned from Heat-not-Burn Tobacco's Past?". Euromonitor International.
- ^ Adriaens, Karolien; Gucht, Dinska Van; Baeyens, Frank (2018). "IQOSTM vs. e-Cigarette vs. Tobacco Cigarette: A Direct Comparison of Short-Term Effects after Overnight-Abstinence". International Journal of Environmental Research and Public Health. 15 (12): 2902. doi:10.3390/ijerph15122902. ISSN 1660-4601. PMID 30567400.
{{cite journal}}: CS1 maint: unflagged free DOI (link) This article incorporates text by Karolien Adriaens, Dinska Van Gucht, and Frank Baeyens available under the CC BY 4.0 license.
This article incorporates text by Karolien Adriaens, Dinska Van Gucht, and Frank Baeyens available under the CC BY 4.0 license.
- ^ Davies, Rob; Monaghan, Angela (30 November 2016). "Philip Morris's vision of cigarette-free future met with scepticism". The Guardian.
- ^ a b Tuinstra, Taco (16 December 2014). "Certified organic e-liquids being launched by VTM". Tobacco Reporter.
- ^ a b O'Connell, Thomas (9 July 2013). "US8479747B2 - Method for preparing tobacco extract for electronic smoking devices". Google Patents.
- ^ a b Staal, Yvonne CM; van de Nobelen, Suzanne; Havermans, Anne; Talhout, Reinskje (2018). "New Tobacco and Tobacco-Related Products: Early Detection of Product Development, Marketing Strategies, and Consumer Interest". JMIR Public Health and Surveillance. 4 (2): e55. doi:10.2196/publichealth.7359. ISSN 2369-2960. PMC 5996176. PMID 29807884.
{{cite journal}}: CS1 maint: unflagged free DOI (link) This article incorporates text by Yvonne CM Staal, Suzanne van de Nobelen, Anne Havermans, and Reinskje Talhout available under the CC BY 4.0 license.
This article incorporates text by Yvonne CM Staal, Suzanne van de Nobelen, Anne Havermans, and Reinskje Talhout available under the CC BY 4.0 license.
- ^ "BAT finds strong Japan demand for its Glo smokeless tobacco device". The Japan Times. Reuters. 22 March 2017.
- ^ a b c Caruana, Diane (25 October 2017). "BAT to launch its HnB device in Russia". VapingPost.
- ^ Caplinger, Dan (31 May 2017). "Here's Why the Worst Might Be Yet to Come for Philip Morris International". The Motley Fool.
- ^ "Innovation Drives BAT's $47 Billion Bid -- WSJ". ADVFN. 24 October 2016.
- ^ a b News Desk (24 October 2016). "World's second largest tobacco company tells people to quit smoking". The Express Tribune.
- ^ Felberbaum, Michael (26 June 2014). "Philip Morris Int'l to Sell Marlboro HeatSticks". Salon (website). Associated Press.
- ^ a b c Gillette, Felix; Kaplan, Jennifer; Chambers, Sam (8 March 2017). "Big Tobacco Has Caught Startup Fever". Bloomberg News.
- ^ Caplinger, Dan (23 November 2015). "5 Things Every Philip Morris Investor Should Know". The Motley Fool.
- ^ Nathan, Ralph (12 October 2016). "Why Philip Morris's iQOS Sales in Japan Are Promising". Market Realist.
- ^ "Philip Morris looks beyond cigarettes with alternative products". Reuters. 30 November 2016.
- ^ a b Kim, Minji (2017). "Philip Morris International introduces new heat-not-burn product, IQOS, in South Korea". Tobacco Control. 27 (e1): tobaccocontrol–2017–053965. doi:10.1136/tobaccocontrol-2017-053965. ISSN 0964-4563. PMC 5966325. PMID 29170165.
- ^ Clarke, Toni (25 January 2018). "U.S. panel deals blow to Philip Morris tobacco device". Reuters.
- ^ Mulier, Thomas; Thesing, Gabi (26 June 2014). "Philip Morris Sees $700 Million Boost From iQOS Smoking Device". Bloomberg News.
- ^ a b Kaplan, Jennifer (19 April 2018). "Philip Morris Plunges the Most in a Decade on Slump in Cigarettes". Bloomberg News.
- ^ Ando, Ritsuko (22 October 2018). "Philip Morris Aims to Revive Japan Sales With Cheaper Heat-Not-Burn Tobacco". U.S. News & World Report.
- ^ Mathers, Annalise; Schwartz, Robert; O'Connor, Shawn; Fung, Michael; Diemert, Lori (2018). "Marketing IQOS in a dark market". Tobacco Control: tobaccocontrol–2017–054216. doi:10.1136/tobaccocontrol-2017-054216. ISSN 0964-4563. PMID 29724866.
- ^ McKelvey, Karma; Popova, Lucy; Kim, Minji; Chaffee, Benjamin W; Vijayaraghavan, Maya; Ling, Pamela; Halpern-Felsher, Bonnie (November 2018). "Heated tobacco products likely appeal to adolescents and young adults". Tobacco Control. 27 (Suppl 1): s41–s47. doi:10.1136/tobaccocontrol-2018-054596. ISSN 0964-4563. PMC 6252490. PMID 30352843.
- ^ "Tobacco company charged over importing prohibited product". The New Zealand Herald. 18 May 2017.
- ^ a b Drope, Jeffrey; Cahn, Zachary; Kennedy, Rosemary; Liber, Alex C.; Stoklosa, Michal; Henson, Rosemarie; Douglas, Clifford E.; Drope, Jacqui (2017). "Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine". CA: A Cancer Journal for Clinicians. 67 (6): 449–471. doi:10.3322/caac.21413. ISSN 0007-9235. PMID 28961314.
- ^ "Philip Morris's Cigarette Alternative Could Hit U.S. in 2017". Bloomberg News. 5 October 2016.
- ^ a b Hendlin, Yogi Hale; Elias, Jesse; Ling, Pamela M. (2017). "The Pharmaceuticalization of the Tobacco Industry". Annals of Internal Medicine. 167 (4): 278–280. doi:10.7326/M17-0759. ISSN 0003-4819. PMC 5568794. PMID 28715843.
- ^ "Philip Morris to invest 300 million euros in Greece for smoke-free product". Reuters. 22 March 2017.
- ^ a b Caplinger, Dan (28 June 2017). "Philip Morris International's Most Brilliant Move So Far in 2017". The Motley Fool.
- ^ Hyo-sik, Lee (17 May 2017). "Philip Morris unveils smoke-free cigarette in Korea". The Korea Times.
- ^ a b Lasseter, Tom; Wilson, Duff; Wilson, Thomas; Bansal, Paritosh (15 May 2018). "Philip Morris device knows a lot about your smoking habit". Reuters.
- ^ a b c d e f g h i j k l m n o p q r s Auer, Reto; Concha-Lozano, Nicolas; Jacot-Sadowski, Isabelle; Cornuz, Jacques; Berthet, Aurélie (1 July 2017). "Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name". JAMA Internal Medicine. 177 (7): 1050–1052. doi:10.1001/jamainternmed.2017.1419. ISSN 2168-6106. PMC 5543320. PMID 28531246.
- ^ Duprey, Rich (9 December 2017). "Will 2018 Be Philip Morris International Inc's Best Year Yet?". Billings Gazette.
- ^ a b Tai, Mariko (31 August 2015). "Philip Morris rolls out iQOS smokeless smokes". Nikkei Asian Review.
- ^ Conquero, Belén V. (20 January 2017). "¿Es posible conseguir un tabaco menos nocivo?" [Is it possible to get a less noxious tobacco?]. La Razón (in Spanish).
- ^ a b Katz, MH; Redberg, RF (6 November 2017). "Science Requires Open Discourse". JAMA Internal Medicine. 178 (1): 15–16. doi:10.1001/jamainternmed.2017.5763. PMID 29114738.
- ^ Kiefer, Bertrand (21 June 2017). "Tabac : nouveau produit, vieilles methods" [Tobacco: New product, Old methods]. Revue Médicale Suisse (in French): 1312.
- ^ a b c William Wan (14 August 2017). "Big tobacco's new cigarette is sleek, smokeless — but is it actually healthier?". The Washington Post.
- ^ "Questions over risks of Philip Morris's smoke-free tobacco device IQOS". CBS News. 24 January 2018.
- ^ a b c d e f "FDA Panel Gives Qualified Support To Claims For". National Public Radio. 25 January 2018.
- ^ a b "Modified Risk Tobacco Product Applications (MRTPAs) MR0000059-MR0000061 Philip Morris Products S.A." (PDF). Food and Drug Administration. 24–25 January 2018. pp. 12–15.
- ^ Lasseter, Tom; Bansal, Paritosh; Wilson, Thomas; Miyazaki, Ami; Wilson, Duff; Kalra, Aditya (20 December 2017). "Scientists describe problems in Philip Morris e-cigarette experiments". Reuters.
- ^ a b Trefis Team (30 December 2016). "FDA Approval For iQOS To Be A Game Changer For Altria". Forbes.
- ^ a b Caplinger, Dan (26 May 2017). "The FDA Moves Forward With Philip Morris iQOS Review". The Motley Fool.
- ^ speccomm (26 May 2017). "FDA starts iQOS review". Tobacco Reporter.
- ^ Chambers, Sam (26 January 2018). "Big Tobacco spending billions to develop products that could move industry beyond cigarettes — but regulators are skeptical". Chicago Tribune. Archived from the original on 21 June 2018.
- ^ "Meeting of the Tobacco Products Scientific Advisory Committee January 24-25, 2018" (PDF). Food and Drug Administration. 24–25 January 2018.
{{cite web}}: CS1 maint: date format (link) - ^ Kaplan, Sheila; Thomas, Katie (11 February 2018). "F.D.A. Chief Goes Against the Administration Stereotype". The New York Times.
- ^ Yui, Monami (28 August 2016). "Big Tobacco Wants to Turn Japan's Smokers Into Vapers". Bloomberg News.
- ^ a b McKelvey, Karma; Popova, Lucy; Kim, Minji; Lempert, Lauren Kass; Chaffee, Benjamin W; Vijayaraghavan, Maya; Ling, Pamela; Halpern-Felsher, Bonnie (2018). "IQOS labelling will mislead consumers". Tobacco Control. 27 (Suppl 1): s48–s54. doi:10.1136/tobaccocontrol-2018-054333. ISSN 0964-4563. PMC 6252493. PMID 30158208.
- ^ Mulier, Thomas; Chambers, Sam; Liefgreen, Liefgreen (24 March 2016). "Marlboro Kicks Some Ash". Bloomberg News.
- ^ "The iSmoke OneHitter, iSmoke "3-in-1" and iSmoke Oven join portfolio". Convenience Store News. 2017.
- ^ a b "Consumer-Centric Vaping". Convenience Store Decisions. Harbor Communications. 4 March 2015.
- ^ a b c d e "The comparison Of The Heat Not Burn Products IUOC and IQOS". AB Newswire. 6 September 2017.
- ^ "KT&G to release heat-not-burn tobacco amid iQOS growing popularity". The Korea Herald. 8 June 2017.
- ^ a b "KT&G launches sales of new tobacco-heating device". Yonhap News Agency. 11 November 2017.
- ^ Yakowicz, Will (10 June 2015). "This Silicon Valley Company Just Raised $47 Million to Smoke Cigarette Makers". Inc.
- ^ Dawson, Freddie (31 July 2015). "Pax Labs Looking At International Expansion". Forbes.
- ^ Biggs, John (17 June 2012). "Smoke Up: An Interview With The Creator Of The Ultracool Pax Vaporizer". TechCrunch.
- ^ Lavrinc, Damon (1 July 2013). "Review: Ploom Model Two". Wired.
- ^ Lawler, Ryan (11 March 2015). "The Pax 2 Improves Upon One Of The Best Vaporizers On The Market". TechCrunch.
- ^ a b Stenovec, Tim (12 March 2016). "How two guys from Stanford built the 'iPhone of vaporizers'". Business Insider.
- ^ Rossel, Stefanie (1 July 2016). "Blending nature and technology". Tobacco Reporter.
- ^ Tuinstra, Taco (26 January 2016). "JT announces launch of next-generation Ploom". Tobacco Reporter.
- ^ Uranaka, Taiga (16 January 2019). "Japan Tobacco ratchets up smokeless war with new products". Reuters.
- ^ "JT to expand Ploom TECH sales". Tobacco Reporter. 2016-10-10.
- ^ Uranaka, Taiga; Sarkar, Himani (29 May 2017). "Japan Tobacco plans to quadruple smokeless tobacco output capacity by 2018: CEO". Channel NewsAsia. Reuters.
- ^ "Imperial Brands aims to convince smokers to switch to vaping products". Sky News. Yahoo! News. 6 November 2018.
- ^ a b c Monkeyshines (1 April 2015). "High Buy: V2 Pro Series 7". High Times.
- ^ a b Kahn, Steven (11 March 2016). "V2 Pro Series 3 Vaporizer Review – Updated for 2017". Bloomberg News.
- ^ a b Silver, Curtis (19 May 2017). "V2 Pro Series 3X Is The Highly Versatile Vape You've Been Looking For". Forbes.
- ^ Craft, Scott (16 June 2015). "V2 Pro Series 7 Review: Premium Vapor Quality From A Surprisingly Affordable Vaporizer". iDigitalTimes.
- ^ Varlet, Vincent; Concha-Lozano, Nicolas; Berthet, Aurélie; Plateel, Grégory; Favrat, Bernard; De Cesare, Mariangela; Lauer, Estelle; Augsburger, Marc; Thomas, Aurélien; Giroud, Christian (2016). "Drug vaping applied to cannabis: Is "Cannavaping" a therapeutic alternative to marijuana?". Scientific Reports. 6 (1): 25599. Bibcode:2016NatSR...625599V. doi:10.1038/srep25599. ISSN 2045-2322. PMC 4881394. PMID 27228348.
- ^ Vu, An T.; Taylor, Kenneth M.; Holman, Matthew R.; Ding, Yan S.; Hearn, Bryan; Watson, Clifford H. (2015). "Polycyclic Aromatic Hydrocarbons in the Mainstream Smoke of Popular U.S. Cigarettes". Chemical Research in Toxicology. 28 (8): 1616–1626. doi:10.1021/acs.chemrestox.5b00190. ISSN 0893-228X. PMC 4540633. PMID 26158771.
- ^ a b c d e f g h i j k l m n o Shi, Yuyan; Caputi, Theodore L.; Leas, Eric; Dredze, Mark; Cohen, Joanna E.; Ayers, John W. (2017). "They're heating up: Internet search query trends reveal significant public interest in heat-not-burn tobacco products". PLOS ONE. 12 (10): e0185735. Bibcode:2017PLoSO..1285735C. doi:10.1371/journal.pone.0185735. ISSN 1932-6203. PMC 5636077. PMID 29020019.
{{cite journal}}: CS1 maint: unflagged free DOI (link) This article incorporates text by Theodore L. Caputi, Eric Leas, Mark Dredze, Joanna E. Cohen, and John W. Ayers available under the CC BY 4.0 license.
This article incorporates text by Theodore L. Caputi, Eric Leas, Mark Dredze, Joanna E. Cohen, and John W. Ayers available under the CC BY 4.0 license.
- ^ McNeill 2018, p. 22, 218.
- ^ Tabuchi, Takahiro; Shinozaki, Tomohiro; Kunugita, Naoki; Nakamura, Masakazu; Tsuji, Ichiro (2018). "Study Profile: The Japan "Society and New Tobacco" Internet Survey (JASTIS): A longitudinal internet cohort study of heat-not-burn tobacco products, electronic cigarettes and conventional tobacco products in Japan". Journal of Epidemiology. doi:10.2188/jea.JE20180116. ISSN 0917-5040. PMID 30318495.
- ^ Liber, Alex C. (2018). "Heated tobacco products and combusted cigarettes: comparing global prices and taxes". Tobacco Control: tobaccocontrol–2018–054602. doi:10.1136/tobaccocontrol-2018-054602. ISSN 0964-4563. PMID 30381439.
- ^ Fisher, Daniel (16 June 2014). "Philip Morris International Bets Big On The Future Of Smoking". Forbes.
- ^ Siegel-Itzkovich, Judy (2 March 2017). "Orgs slam Litzman for allowing sale of iQOS heated, smokeless cigarettes". The Jerusalem Post.
- ^ Ronny Linder-Ganz (16 January 2018). "In Blow to Philip Morris, Israel to Tax iQOS E-cigarettes Like Ordinary Cigarettes". Haaretz.
- ^ Judy Siegel-Itzkovich (3 April 2018). "Justice Ministry says iQOS product will be treated as ordinary tobacco". The Jerusalem Post.
- ^ Tabuchi, Takahiro; Kiyohara, Kosuke; Hoshino, Takahiro; Bekki, Kanae; Inaba, Yohei; Kunugita, Naoki (2016). "Awareness and use of electronic cigarettes and heat-not-burn tobacco products in Japan". Addiction. 111 (4): 706–713. doi:10.1111/add.13231. ISSN 0965-2140. PMID 26566956.
- ^ "Tax hike on heat-not-burn tobacco products under consideration as LDP begins review of tax reforms". The Japan Times. 8 September 2017.
- ^ Jae-hyuk, Park (26 May 2017). "IQOS available in Seoul Saturday". The Korea Times.
- ^ a b Trefis Team (13 September 2017). "Why Is Korea Easier To Conquer For iQOS Than Europe?". NASDAQ.
- ^ "Singapore Enhances Tobacco Control Measures". Ministry of Health (Singapore). 28 July 2016.
- ^ Elder, Vaughan (13 January 2017). "Legality of tobacco product in question". Otago Daily Times.
- ^ Caruana, Diane (3 February 2017). "iQos heatsticks declared illegal in NZ". VapingPost.
- ^ a b "New Zealand's legal action against IQOS postponed, consultation with Big Tobacco follows". 130 (1465). New Zealand Medical Journal. 10 November 2017.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "iQOS may not be as harm-free as claimed, study finds". The New Zealand Herald. 14 March 2018.
- ^ Zaharia, Marius (27 March 2018). "New Zealand court gives Philip Morris nod to sell heated tobacco product". Reuters.
- ^ MHNZ 2017, p. ii.