2601:643:8100:8af4:e913:d0df:e7ab:d943 (talk) |
Undid revision 684605250 by 2601:643:8100:8AF4:E913:D0DF:E7AB:D943 (talk) |
||
| Line 71: | Line 71: | ||
==Adverse effects== |
==Adverse effects== |
||
As with any anticoagulant the most serious adverse effect is [[bleeding]], including severe [[internal bleeding]].<ref>{{cite web|title=Medication Guide--Xarelto|url=http://www.fda.gov/downloads/Drugs/DrugSafety/UCM280333.pdf|website=http://www.fda.gov/|publisher=U.S. Food and Drug Administration|accessdate=1 September 2014}}</ref><ref>{{cite web|title=Xarelto Side Effects|url=http://www.webmd.com/drugs/2/drug-156265/xarelto-oral/details/list-sideeffects|website=http://www.webmd.com/|publisher=WebMD|accessdate=1 September 2014}}</ref><ref>{{cite web|title=Xarelto Side Effects Center|url=http://www.rxlist.com/xarelto-side-effects-drug-center.htm|website=http://www.rxlist.com/|publisher=RxList|accessdate=1 September 2014}}</ref> There is currently no antidote for rivaroxaban (unlike warfarin, the action of which can be reversed with [[vitamin K]] or [[prothrombin complex concentrate]]), meaning that serious bleeding may be difficult to manage. A possible antidote ([[andexanet alfa]]) is being investigated.<ref>{{cite journal | title=Recent advances in the development of specific antidotes for target-specific oral anticoagulants | author=Mo Y, Yam FK | journal=Pharmacotherapy | date=Feb 2015 | volume=35 | issue=2 | pages=198–207 | doi=10.1002/phar.1532 | pmid=25644580}}</ref> |
As with any anticoagulant the most serious adverse effect is [[bleeding]], including severe [[internal bleeding]].<ref>{{cite web|title=Medication Guide--Xarelto|url=http://www.fda.gov/downloads/Drugs/DrugSafety/UCM280333.pdf|website=http://www.fda.gov/|publisher=U.S. Food and Drug Administration|accessdate=1 September 2014}}</ref><ref>{{cite web|title=Xarelto Side Effects|url=http://www.webmd.com/drugs/2/drug-156265/xarelto-oral/details/list-sideeffects|website=http://www.webmd.com/|publisher=WebMD|accessdate=1 September 2014}}</ref><ref>{{cite web|title=Xarelto Side Effects Center|url=http://www.rxlist.com/xarelto-side-effects-drug-center.htm|website=http://www.rxlist.com/|publisher=RxList|accessdate=1 September 2014}}</ref> There is currently no antidote for rivaroxaban (unlike warfarin, the action of which can be reversed with [[vitamin K]] or [[prothrombin complex concentrate]]), meaning that serious bleeding may be difficult to manage. A possible antidote ([[andexanet alfa]]) is being investigated.<ref>{{cite journal | title=Recent advances in the development of specific antidotes for target-specific oral anticoagulants | author=Mo Y, Yam FK | journal=Pharmacotherapy | date=Feb 2015 | volume=35 | issue=2 | pages=198–207 | doi=10.1002/phar.1532 | pmid=25644580}}</ref> Xarelto is currently indicated as an high-risk medication, due to the lack of antidote and the signifcant risk of severe side effects such as spontaneous spinal epidural hematoma, intracranial and gastrointestinal bleeding. The ROCKET-AF double-blind trial that allegedly demonstrated Xarelto's superiority in terms of safety and effectiveness over Warfarin, was recently contested by FDA reviewers, who examined the data from the trial also by pointing out how Warfarin was administered in a sub-optimal way<ref>{{Cite web|url = https://www.xareltocares.com/xarelto-a-truly-dangerous-medication/|title = Xarelto Side Effects: a truly dangerous medication|date = October 2015|accessdate = October 2015|website = Xarelto Lawsuit Information - Blood Thinner Uses & Bleeding Side Effects|publisher = |last = Dr. Butticè|first = Claudio}}</ref>. Clinical evidence from the [http://www.ismp.org/ Institute for Safe Medication Practices (ISMP)] pointed out that Xarelto can also be associated to an increased risk for blood clot-related injuries such as pulmonary edema or venous thromboembolism<ref>{{Cite web|url = http://www.ismp.org/quarterwatch/pdfs/2012Q1.pdf|title = QuarterWatch Monitoring FDA MedWatch Reports – Why Reports of Serious Adverse Drug Events Continue to Grow – October 3, 2012 – Data from 2012 Quarter 1|date = October 3, 2012|accessdate = October 2015|website = Institute for Safe Medication Practices (ISMP)|publisher = |last = |first = }}</ref>. |
||
==Mechanism of action== |
==Mechanism of action== |
||
Revision as of 17:59, 7 October 2015
{{Drugbox
| Verifiedfields = changed
| verifiedrevid = 459434616
| IUPAC_name = (S)-5-chloro-N-{[2-oxo-3-[4-(3-oxomorpholin-4-yl)
phenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide
| image = Rivaroxaban2DCSD.svg
| width = 250px
| image2 = Rivaroxaban xtal 2005.png
| width2 = 250px
| tradename = Xarelto | Drugs.com = Micromedex Detailed Consumer Information | licence_EU = Xarelto | licence_US = Rivaroxaban | pregnancy_AU = C | pregnancy_US = C | legal_AU = S4 | legal_CA = Rx-only | legal_UK = POM | legal_US = Rx-only | legal_status = | routes_of_administration = oral
| bioavailability = 80% to 100%; Cmax = 2 – 4 hours (10 mg oral)[1] | metabolism = CYP3A4 , CYP2J2 and CYP-independent mechanisms[1] | elimination_half-life = 5 – 9 hours in healthy subjects aged 20 to 45[1][2] | excretion = 2/3 metabolized in liver and 1/3 eliminated unchanged[1]
| IUPHAR_ligand = 6388
| CAS_number_Ref = ![]() | CAS_number = 366789-02-8
| ATC_prefix = B01
| ATC_suffix = AX06
| PubChem = 6433119
| DrugBank_Ref =
| CAS_number = 366789-02-8
| ATC_prefix = B01
| ATC_suffix = AX06
| PubChem = 6433119
| DrugBank_Ref = ![]() | DrugBank = DB06228
| ChemSpiderID_Ref =
| DrugBank = DB06228
| ChemSpiderID_Ref = ![]() | ChemSpiderID = 8051086
| UNII_Ref =
| ChemSpiderID = 8051086
| UNII_Ref = ![]() | UNII = 9NDF7JZ4M3
| ChEMBL_Ref =
| UNII = 9NDF7JZ4M3
| ChEMBL_Ref = ![]() | ChEMBL = 198362
| synonyms = Xarelto, BAY 59-7939
| ChEMBL = 198362
| synonyms = Xarelto, BAY 59-7939
| C=19 | H=18 | Cl=1 | N=3 | O=5 | S=1
| molecular_weight = 435.882 g/mol
| smiles = c1cc(ccc1N2CCOCC2=O)N3C[C@@H](OC3=O)CNC(=O)c4ccc(s4)Cl
| InChI = 1/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
| InChIKey = KGFYHTZWPPHNLQ-AWEZNQCLBK
| StdInChI_Ref = ![]() | StdInChI = 1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
| StdInChIKey_Ref =
| StdInChI = 1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
| StdInChIKey_Ref = ![]() | StdInChIKey = KGFYHTZWPPHNLQ-AWEZNQCLSA-N
}}
| StdInChIKey = KGFYHTZWPPHNLQ-AWEZNQCLSA-N
}}
Rivaroxaban (BAY 59-7939) is an oral anticoagulant invented and manufactured by Bayer;[3][4] in a number of countries it is marketed as Xarelto.[1] In the United States, it is marketed by Janssen Pharmaceutica.[5] It is the first available orally active direct factor Xa inhibitor. Rivaroxaban is well absorbed from the gut and maximum inhibition of factor Xa occurs four hours after a dose. The effects last approximately 8–12 hours, but factor Xa activity does not return to normal within 24 hours so once-daily dosing is possible.
Medical uses
In those with non-valvular atrial fibrillation it appears to be as effective as warfarin in preventing nonhemorrhagic strokes and embolic events.[6] Rivaroxaban is associated with lower rates of serious and fatal bleeding events than warfarin though rivaroxaban is associated with higher rates of bleeding in the gastrointestinal tract.[7]
In September 2008, Health Canada granted marketing authorization for rivaroxaban for the prevention of venous thromboembolism (VTE) in people who have undergone elective total hip replacement or total knee replacement surgery.[8]
In September 2008, the European Commission granted marketing authorization of rivaroxaban for the prevention of venous thromboembolism in adults undergoing elective hip and knee replacement surgery.[9]
On July 1, 2011, the U.S. Food and Drug Administration (FDA) approved rivaroxaban for prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in adults undergoing hip and knee replacement surgery.[5]
On November 4, 2011, the U.S. FDA approved rivaroxaban for stroke prophylaxis in people with non-valvular atrial fibrillation.
Adverse effects
As with any anticoagulant the most serious adverse effect is bleeding, including severe internal bleeding.[10][11][12] There is currently no antidote for rivaroxaban (unlike warfarin, the action of which can be reversed with vitamin K or prothrombin complex concentrate), meaning that serious bleeding may be difficult to manage. A possible antidote (andexanet alfa) is being investigated.[13] Xarelto is currently indicated as an high-risk medication, due to the lack of antidote and the signifcant risk of severe side effects such as spontaneous spinal epidural hematoma, intracranial and gastrointestinal bleeding. The ROCKET-AF double-blind trial that allegedly demonstrated Xarelto's superiority in terms of safety and effectiveness over Warfarin, was recently contested by FDA reviewers, who examined the data from the trial also by pointing out how Warfarin was administered in a sub-optimal way[14]. Clinical evidence from the Institute for Safe Medication Practices (ISMP) pointed out that Xarelto can also be associated to an increased risk for blood clot-related injuries such as pulmonary edema or venous thromboembolism[15].
Mechanism of action
Rivaroxaban is an oxazolidinone derivative optimized for inhibiting both free Factor Xa and Factor Xa bound in the prothrombinase complex.[3] It is a highly selective direct Factor Xa inhibitor with oral bioavailability and rapid onset of action. Inhibition of Factor Xa interrupts the intrinsic and extrinsic pathway of the blood coagulation cascade, inhibiting both thrombin formation and development of thrombi. Rivaroxaban does not inhibit thrombin (activated Factor II), and no effects on platelets have been demonstrated.[1]
Rivaroxaban has predictable pharmacokinetics across a wide spectrum of patients (age, gender, weight, race) and has a flat dose response across an eightfold dose range (5–40 mg).[16] Clinical trial data have shown that it allows predictable anticoagulation with no need for dose adjustments and routine coagulation monitoring.[1] However, these trials have excluded people with significant liver disease and end-stage kidney disease; therefore, the safety of rivaroxaban in these populations is unknown.
Chemistry
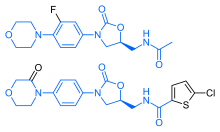
Rivaroxaban bears a striking structural similarity to the antibiotic linezolid: both drugs share the same oxazolidinone-derived core structure. Accordingly, rivaroxaban was studied for any possible antimicrobial effects and for the possibility of mitochondrial toxicity, which is a known complication of long-term linezolid use. Studies found that neither rivaroxaban nor its metabolites have any antibiotic effect against Gram-positive bacteria. As for mitochondrial toxicity, in vitro studies found the risk to be low.[17]
Related drugs
A number of anticoagulants inhibit the activity of Factor Xa. Unfractionated heparin (UFH), low molecular weight heparin (LMWH), and fondaparinux inhibit the activity of factor Xa indirectly by binding to circulating antithrombin (AT III). These agents must be injected. Warfarin, phenprocoumon, and acenocoumarol are orally active vitamin K antagonists (VKA) which decrease the liver's production of a number of coagulation factors, including Factor X. In recent years, a new series of oral, direct acting inhibitors of Factor Xa have entered clinical development. These include rivaroxaban, apixaban, betrixaban, LY517717, darexaban (YM150), and edoxaban (DU-176b).[18]
Research
In October 2014, Portola Pharmaceuticals announced the completion of Phase I and Phase II clinical trials for andexanet alfa as an antidote for Factor Xa inhibitors with few adverse effects, as well as the start of Phase III trials. The compound has yet to be approved by the U.S. Food and Drug Administration.[19]
References
- ^ a b c d e f g "Xarelto: Summary of Product Characteristics". Bayer Schering Pharma AG. 2008. Retrieved 2009-02-11.
- ^ Abdulsattar, Y; Bhambri, R; Nogid, A (May 2009). "Rivaroxaban (xarelto) for the prevention of thromboembolic disease: an inside look at the oral direct factor xa inhibitor". P & T : a peer-reviewed journal for formulary management. 34 (5): 238–44. PMID 19561868.
- ^ a b Roehrig S, Straub A, Pohlmann J, et al. (September 2005). "Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor". Journal of Medicinal Chemistry. 48 (19): 5900–8. doi:10.1021/jm050101d. PMID 16161994.
- ^ Perzborn, Elisabeth; Roehrig, Susanne; Straub, Alexander; Kubitza, Dagmar; Misselwitz, Frank (17 December 2010). "The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor". Nature Reviews Drug Discovery. 10 (1): 61–75. doi:10.1038/nrd3185.
- ^ a b "FDA Approves XARELTO® (rivaroxaban tablets) to Help Prevent Deep Vein Thrombosis in Patients Undergoing Knee or Hip Replacement Surgery" (Press release). Janssen Pharmaceutica. 2011-07-01. Retrieved 2011-07-01.
- ^ Gómez-Outes, A; Terleira-Fernández, AI; Calvo-Rojas, G; Suárez-Gea, ML; Vargas-Castrillón, E (2013). "Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups". Thrombosis. 2013: 640723. doi:10.1155/2013/640723. PMC 3885278. PMID 24455237.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Brown DG, Wilkerson EC, Love WE (March 2015). "A review of traditional and novel oral anticoagulant and antiplatelet therapy for dermatologists and dermatologic surgeons". Journal of the American Academy of Dermatology. 72 (3): 524–34. doi:10.1016/j.jaad.2014.10.027. PMID 25486915.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Bayer's Xarelto Approved in Canada" (Press release). Bayer. 2008-09-16. Retrieved 2010-01-31.
- ^ "Bayer's Novel Anticoagulant Xarelto now also Approved in the EU" (Press release). Bayer. 2008-02-10. Retrieved 2010-01-31.
- ^ "Medication Guide--Xarelto" (PDF). http://www.fda.gov/. U.S. Food and Drug Administration. Retrieved 1 September 2014.
{{cite web}}: External link in|website= - ^ "Xarelto Side Effects". http://www.webmd.com/. WebMD. Retrieved 1 September 2014.
{{cite web}}: External link in|website= - ^ "Xarelto Side Effects Center". http://www.rxlist.com/. RxList. Retrieved 1 September 2014.
{{cite web}}: External link in|website= - ^ Mo Y, Yam FK (Feb 2015). "Recent advances in the development of specific antidotes for target-specific oral anticoagulants". Pharmacotherapy. 35 (2): 198–207. doi:10.1002/phar.1532. PMID 25644580.
- ^ Dr. Butticè, Claudio (October 2015). "Xarelto Side Effects: a truly dangerous medication". Xarelto Lawsuit Information - Blood Thinner Uses & Bleeding Side Effects. Retrieved October 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "QuarterWatch Monitoring FDA MedWatch Reports – Why Reports of Serious Adverse Drug Events Continue to Grow – October 3, 2012 – Data from 2012 Quarter 1" (PDF). Institute for Safe Medication Practices (ISMP). October 3, 2012. Retrieved October 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Eriksson BI, Borris LC, Dahl OE, et al. (November 2006). "A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement". Circulation. 114 (22): 2374–81. doi:10.1161/CIRCULATIONAHA.106.642074. PMID 17116766.
- ^ European Medicines Agency (2008). "CHP Assessment Report for Xarelto (EMEA/543519/2008)" (PDF). Retrieved 2009-06-11.
- ^ Turpie AG (January 2008). "New oral anticoagulants in atrial fibrillation". European Heart Journal. 29 (2): 155–65. doi:10.1093/eurheartj/ehm575. PMID 18096568.
- ^ Schroeder, C. "Possible Antidote Could Help Blood Thinner Patients In Bleeding Emergencies". DrugNews. Retrieved 20 August 2015.