| |
| Names | |
|---|---|
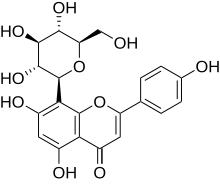
| IUPAC name
8-(β-D-Glucopyranosyl)-4′,5,7-trihydroxyflavone
| |
| Systematic IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-4H-1-benzopyran-4-one | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.876 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H20O10 | |
| Molar mass | 432.38 g/mol |
| Appearance | Light yellow powder |
| Melting point | 203 to 204 °C (397 to 399 °F; 476 to 477 K) |
| Supplementary data page | |
| Vitexin (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Vitexin is an apigenin flavone glucoside, a chemical compound found in the passion flower, Vitex agnus-castus (chaste tree or chasteberry), in the Phyllostachys nigra bamboo leaves,[1] in the pearl millet (Pennisetum millet),[2] and in Hawthorn.[3]
Metabolism[edit]
Goitrogenicity of millet flavones : Vitexin inhibits thyroid peroxidase thus contributing to goiter.[4][5]
See also[edit]
- Isovitexin (or homovitexin, saponaretin) is the apigenin-6-C-glucoside.
- Orientin, the 3'-OH derivative
References[edit]
- ^ Zhang Y, Jiao J, Liu C, Wu X, Zhang Y (2007). "Isolation and purification of four flavone C-glycosides from antioxidant of bamboo leaves by macroporous resin column chromatography and preparative high-performance liquid chromatography". Food Chemistry. doi:10.1016/j.foodchem.2007.09.037. Archived from the original on 2012-09-10.
- ^ J.O. AKINGBALA (1991). "Effect of Processing on Flavonoids in Millet (Pennisetum americanum) Flour" (PDF). Cereal Chem. 68 (2): 180–183. Archived from the original (PDF) on 2009-05-14. Retrieved 2009-08-21.
- ^ Scholz H (1995). Gustav Hegi. Illustrierte Flora von Mitteleuropa IV(2B). Spermatophyta: Angiospermae: Dicotyledones 2(3). Rosaceae 2 (2nd ed.). Berlin: Blackwell Wissenschafts-Verlag. p. 431. ISBN 978-3-8263-2533-5.
- ^ Gaitan E (1990). "Goitrogens in food and water". Annual Review of Nutrition. 10: 21–39. doi:10.1146/annurev.nu.10.070190.000321. PMID 1696490.
- ^ Birzer DM, Klopfenstein CF, Leipold HW (1987). "Goitre causing compounds found in pearl millet". Nutr. Rep. Int. 36: 131–141. ISSN 0029-6635.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction