 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
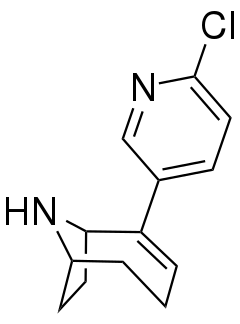
| Formula | C13H15ClN2 |
| Molar mass | 234.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
UB-165 is a drug which acts as an agonist at neuronal nicotinic acetylcholine receptors being a full agonist of the α3β2 isoform and a partial agonist of the α4β2* isoform. It is used to study the role of this receptor subtype in the release of dopamine and noradrenaline in the brain,[1][2] and has also been used as a lead compound to derive a number of other selective nicotinic receptor ligands.[3][4][5][6]
References[edit]
- ^ Sharples CG, Kaiser S, Soliakov L, Marks MJ, Collins AC, Washburn M, et al. (April 2000). "UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes". The Journal of Neuroscience. 20 (8): 2783–91. doi:10.1523/JNEUROSCI.20-08-02783.2000. PMC 6772190. PMID 10751429.
- ^ Cao YJ, Surowy CS, Puttfarcken PS (March 2005). "Nicotinic acetylcholine receptor-mediated [3H]dopamine release from hippocampus". The Journal of Pharmacology and Experimental Therapeutics. 312 (3): 1298–304. doi:10.1124/jpet.104.076794. PMID 15542623. S2CID 20437091.
- ^ Gohlke H, Gündisch D, Schwarz S, Seitz G, Tilotta MC, Wegge T (February 2002). "Synthesis and nicotinic binding studies on enantiopure diazine analogues of the novel (2-chloro-5-pyridyl)-9-azabicyclo[4.2.1]non-2-ene UB-165". Journal of Medicinal Chemistry. 45 (5): 1064–72. doi:10.1021/jm010936y. PMID 11855986.
- ^ Sharples CG, Karig G, Simpson GL, Spencer JA, Wright E, Millar NS, et al. (July 2002). "Synthesis and pharmacological characterization of novel analogues of the nicotinic acetylcholine receptor agonist (+/-)-UB-165". Journal of Medicinal Chemistry. 45 (15): 3235–45. doi:10.1021/jm020814l. PMID 12109907.
- ^ Sutherland A, Gallagher T, Sharples CG, Wonnacott S (March 2003). "Synthesis of two fluoro analogues of the nicotinic acetylcholine receptor agonist UB-165". The Journal of Organic Chemistry. 68 (6): 2475–8. doi:10.1021/jo026698b. PMID 12636420.
- ^ Karig G, Large JM, Sharples CG, Sutherland A, Gallagher T, Wonnacott S (September 2003). "Synthesis and nicotinic binding of novel phenyl derivatives of UB-165. Identifying factors associated with alpha7 selectivity". Bioorganic & Medicinal Chemistry Letters. 13 (17): 2825–8. doi:10.1016/S0960-894X(03)00594-8. PMID 14611837.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction