
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| Properties | |
| P3Sn | |
| Molar mass | 211.631 g·mol−1 |
| Appearance | black solid |
| Density | 4.25 g/cm3 |
| Melting point | 580 °C (1,076 °F; 853 K) |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin triphosphide is a binary inorganic compound of tin metal and phosphorus with the chemical formula SnP3.[1]
Structure[edit]
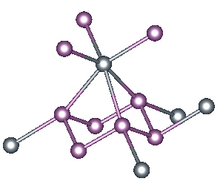
X-ray crystallography reveals that tin triphosphide is not a triphosphide. It is a hexaphosphide, with P66- rings. These ruffled P6 rings form three short (2.66 Å) and three long (2.95 Å) Sn-P bonds. The result is that Sn(II) adopts highly distorted octahedral geometry. The structure of tin triphosphide resembles that of gray arsenic, which also features corrugated, linked six-membered (As6) rings, wherein each arsenic atom has a highly distorted octahedral geometry. Germanium triphosphide and tin triphosphide are similar structurally as well.
Tin triphosphide forms triclinic crystals, spatial group R3m with six formula units in a unit cell of dimensions a = 7.378 Å and c = 10.512 Å.[2][3]
Preparation and occurrence[edit]
Tin triphosphide can be formed from the fusion of stoichiometric amounts of both elements at 580 °C:
- Sn + 3P → SnP3
SnP3 has been evaluated for use in energy storage devices.[4]
Related compounds[edit]
References[edit]
- ^ Jacobson, Carl Alfred; Hampel, Clifford A. (1946). Encyclopedia of Chemical Reactions. Reinhold Publishing Corporation. p. 15. Retrieved 28 March 2024.
- ^ Gullman, Jan; Olofsson, Olle (1 November 1972). "The crystal structure of SnP3 and a note on the crystal structure of GeP3". Journal of Solid State Chemistry. 5 (3): 441–445. doi:10.1016/0022-4596(72)90091-6. ISSN 0022-4596. Retrieved 28 March 2024.
- ^ "mp-7541: SnP3 (trigonal, R-3m, 166)". Materials Project. Retrieved 28 March 2024.
- ^ Yan, Miaomiao; Yang, Bingchao; Sun, Xiujie; Wang, Zhixiu; Jiang, Xingang; Yi, Wencai; Sun, Hairui; Yang, Ruilong; Ding, Hao; Yue, Dongdong; Zhai, Kun; Li, Yueqing; Chen, Xin; Zhang, Yongsheng; Liu, Xiaobing (1 January 2024). "High-Quality 2D SnP3 Nanosheets: Novel Flexible Electrode for Energy Storage Device Application with Wide Temperature Adaptivity". ACS Materials Letters. 6 (1): 194–202. doi:10.1021/acsmaterialslett.3c01438. ISSN 2639-4979. Retrieved 28 March 2024.
- ^ Donohue, Paul C. (1970). "Synthesis, Structure, and Superconducting Properties of New High-Pressure Forms of Tin Phosphide". Inorganic Chemistry. 9 (2): 335–337. doi:10.1021/ic50084a032.
- ^ Olofsson, Olle; Jerslev, Bodil; Thom, Erling; Stoll, E.; Eriksson, G.; Blinc, R.; Paušak, S.; Ehrenberg, L.; Dumanović, J. (1967). "On the Crystal Structure of Sn4P3". Acta Chemica Scandinavica. 21: 1659–1660. doi:10.3891/acta.chem.scand.21-1659.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction