| |
| Names | |
|---|---|
| Preferred IUPAC name
Hydroxypropanedioic acid | |
| Other names
tartronic acid,
2-tartronic acid, hydroxymalonic acid, 2-hydroxymalonic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.184 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H4O5 | |
| Molar mass | 120.06 g/mol |
| Appearance | white solid |
| Density | 1.849 g/cm3 |
| Melting point | 159 °C (318 °F; 432 K) (decomposes) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related carboxylic acids
|
Tartaric acid Malic acid Mesoxalic acid Lactic acid 3-Hydroxypropionic acid Malonic acid Propionic acid Oxalic acid |
Related compounds
|
Glyceric acid Glyceraldehyde Tartonaldehyde Glycerol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
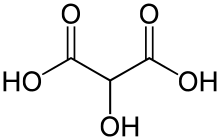
Tartronic acid or 2-hydroxymalonic acid is an organic compound with the structural formula of HOHC(CO2H)2. This dicarboxylic acid is related to malonic acid. It is a white solid. It is produced by oxidation of glycerol:
- HOCH2CH(OH)CH2OH + 2 O2 → HO2CCH(OH)CO2H + 2 H2O
Glyceric acid HOCH2CH(OH)CO2H is an intermediate.[1][2]
Its derivative, 2-methyltartronic acid, is isomalic acid.[3]
Uses[edit]
Oxidation of tartronic acid gives the ketone mesoxalic acid, the simplest oxodicarboxylic acid.[4]
References[edit]
- ^ Habe, Hiroshi; Fukuoka, Tokuma; Kitamoto, Dai; Sakaki, Keiji (2009). "Biotechnological production of d-glyceric acid and its application". Applied Microbiology and Biotechnology. 84 (3): 445–452. doi:10.1007/s00253-009-2124-3. PMID 19621222. S2CID 9144557.
- ^ Yang, Lihua; Li, Xuewen; Chen, Ping; Hou, Zhaoyin (2019). "Selective oxidation of glycerol in a base-free aqueous solution: A short review". Chinese Journal of Catalysis. 40 (7): 1020–1034. doi:10.1016/S1872-2067(19)63301-2. S2CID 196894235.
- ^ Roelofsen, G.; Kanters, J. A.; Kroon, J.; Doesburg, H. M.; Koops, T. (1978). "Order–disorder phenomena in structures of carboxylic acids: The structures of fluoromalonic acid and hydroxymalonic acid at 20 and –150°C". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 34 (8): 2565–2570. Bibcode:1978AcCrB..34.2565R. doi:10.1107/S0567740878008596.
- ^ Fordham P.; Besson M.; Gallezot P. (1997). "Catalytic oxidation with air of tartronic acid to mesoxalic acid on bismuth-promoted platinum". Catal. Lett. 46 (3–4): 195–199(5). doi:10.1023/A:1019082905366. S2CID 92764231. Retrieved 2007-07-06.
- Hall A. N.; Kulka D.; Walker T. K. (1955). "Formation of arabinose, ribulose and tartronic acid from 2-keto-d-gluconic acid". Biochem. J. 60 (2): 271–274(4). doi:10.1042/bj0600271. PMC 1215693. PMID 14389236.
External links[edit]
- US-Patent 4319045: "Process for production of a tartronic acid solution", max 20% Tartronic acid besides other dicarbonic acids
- US-Patent 5750037: Use of tartronic acid as an oxygen scavenger
- Literature overview about synthesis

Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction