| |||
| Names | |||
|---|---|---|---|
| IUPAC name
nitrogen tribromide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NBr3 | |||
| Molar mass | 253.7187 g/mol | ||
| Appearance | Deep red solid | ||
| Melting point | Explodes at -100 °C[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Nitrogen tribromide is a chemical compound with the formula NBr3. It is extremely explosive in its pure form, even at −100 °C, and was not isolated until 1975.[2] It is a deep-red and volatile solid.
Preparation
[edit]NBr3 was first prepared by reaction of bistrimethylsilylbromamine (bis(trimethylsilyl)amine bromide) with bromine monochloride (with trimethylsilyl chloride as byproduct) at −87 °C according to the following equation:
- (Me3Si)2NBr + 2 BrCl → NBr3 + 2 Me
3SiCl
where "Me" is a methyl group.
NBr3 can be produced by the reaction of bromine or hypobromite and ammonia in a dilute aqueous buffer solution.[3] It can also be prepared by the reaction of bromine and bromine azide.[4] Ammonia and bromine undergo glow discharge, and after treatment, red NBr3·6NH3 can be obtained.[5] Pure nitrogen NBr3 was only produced in 1975.[6]
Reactions
[edit]Nitrogen tribromide reacts instantly with ammonia in dichloromethane solution at −87 °C to yield NBrH2.[7]
- NBr3 + 2 NH3 → 3 NH2Br
It also reacts with iodine in dichloromethane solution at −87 °C to produce NBr2I, which is a red-brown solid that stable up to -20 °C.[7]
- NBr3 + I2 → NBr2I + IBr
References
[edit]- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–73, ISBN 0-8493-0594-2
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 439. ISBN 978-0-08-037941-8.
- ^ Galal-Gorchev, Hend; Morris, J. Carrell (Jun 1965). "Formation and Stability of Bromamide, Bromimide, and Nitrogen Tribromide in Aqueous Solution". Inorganic Chemistry. 4 (6): 899–905. doi:10.1021/ic50028a029. ISSN 0020-1669.
- ^ Klapötke, Thomas M. (1997-01-01). "The reaction of bromine azide with bromine". Polyhedron. 16 (15): 2701–2704. doi:10.1016/S0277-5387(96)00586-4. ISSN 0277-5387.
- ^ Schmeisser, Martin (1941-05-07). "Über Bromstickstoff". Zeitschrift für anorganische und allgemeine Chemie. 246 (3): 284–302. doi:10.1002/zaac.19412460305. ISSN 0863-1786.
- ^ Jander, Jochen; Knackmuss, Jürgen; Thiedemann, Klaus-Ulrich (1975-06-01). "Notizen: Darstellung und Isolierung von Stickstofftribromid NBr3 und Stickstoffdibromidmonojodid NBr2J / Preparation and Isolation of Nitrogentribromide NBr3 and Nitrogendibromidemonoiodide NBr2I". Zeitschrift für Naturforschung B. 30 (5–6): 464–465. doi:10.1515/znb-1975-5-633. ISSN 1865-7117.
- ^ a b Matyáš, Robert; Pachman, Jiří. (2013). Primary explosives. Berlin: Springer. p. 294. ISBN 978-3-642-28436-6. OCLC 832350093.
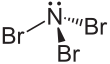

Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction