| |
| Names | |
|---|---|
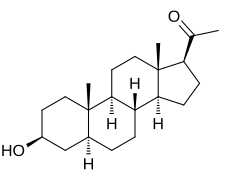
| IUPAC name
3β-Hydroxy-5α-pregnan-20-one
| |
| Systematic IUPAC name
1-[(1S,3aS,3bR,5aS,7S,9aS,9bS,11aS)-7-Hydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]ethan-1-one | |
| Other names
Isoallopregnanolone; Epiallopregnanolone; Sepranolone; 3β,5α-Tetrahydroprogesterone; 3β,5α-THP
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.478 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H34O2 | |
| Molar mass | 318.49 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isopregnanolone, also known as isoallopregnanolone and epiallopregnanolone, as well as sepranolone (INN), and as 3β-hydroxy-5α-pregnan-20-one or 3β,5α-tetrahydroprogesterone (3β,5α-THP), is an endogenous neurosteroid and a natural 3β-epimer of allopregnanolone.[1][2] It has been reported to act as a subunit-selective negative allosteric modulator of the GABAA receptor,[2] and antagonizes in animals and humans some but not all of the GABAA receptor-mediated effects of allopregnanolone, such as anesthesia,[3] sedation,[4] and reduced saccadic eye movements,[4] but not learning impairment.[2] Isopregnanolone has no hormonal effects and appears to have no effect on the GABAA receptor by itself; it selectively antagonizes allopregnanolone and does not affect the effects of other types of GABAA receptor positive allosteric modulators such as benzodiazepines or barbiturates.[1][5]
Isopregnanolone is synthesized from progesterone in the body by the actions of the enzymes 5α-reductase and 3β-hydroxysteroid dehydrogenase (with 5α-dihydroprogesterone as the intermediate in this two-step transformation)[6] and can be reversibly metabolized into allopregnanolone by the enzyme 3α-hydroxysteroid dehydrogenase.[1][2] Levels of isopregnanolone, progesterone, and allopregnanolone are highly correlated across the menstrual cycle and throughout pregnancy.[1] The concentrations of isopregnanolone are significantly less than those of progesterone and allopregnanolone; about half of those of allopregnanolone, to be precise.[6] Isopregnanolone has a relatively long serum elimination half-life of 14 hours in humans.[1]
Isopregnanolone (developmental code name UC-1010) is under development for the treatment of premenstrual dysphoric disorder.[7][8] As of 2017, it is in phase II clinical trials for this indication.[7][8]
Chemistry[edit]
See also[edit]
References[edit]
- ^ a b c d e Hedström H, Bixo M, Nyberg S, Spigset O, Zingmark E, Bäckström T (2009). "Studies of pharmacokinetic and pharmacodynamic properties of isoallopregnanolone in healthy women". Psychopharmacology. 203 (1): 85–98. doi:10.1007/s00213-008-1372-8. PMID 18949461. S2CID 720976.
- ^ a b c d Öfverman, C., Strömberg, J., Birzniece, V., Turkmen, S., Hill, M., Lundgren, P., ... & Johansson, I. M. (2009). The progesterone metabolite isoallopregnanolone is a subunit-selective antagonist of the GABA-A receptor. Chicago
- ^ Bäckström T, Wahlström G, Wahlström K, Zhu D, Wang MD (2005). "Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats". Eur. J. Pharmacol. 512 (1): 15–21. doi:10.1016/j.ejphar.2005.01.049. PMID 15814085.
- ^ a b Bengtsson SK, Nyberg S, Hedström H, Zingmark E, Jonsson B, Bäckström T, Bixo M (2015). "Isoallopregnanolone antagonize allopregnanolone-induced effects on saccadic eye velocity and self-reported sedation in humans". Psychoneuroendocrinology. 52: 22–31. doi:10.1016/j.psyneuen.2014.10.025. PMID 25459890. S2CID 25703203.
- ^ Lundgren, Per; Strömberg, Jessica; Bäckström, Torbjörn; Wang, Mingde (2003). "Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3β-hydroxy-5α-pregnan-20-one (isoallopregnanolone)". Brain Research. 982 (1): 45–53. doi:10.1016/S0006-8993(03)02939-1. ISSN 0006-8993. PMID 12915239. S2CID 54297803.
- ^ a b Abraham Weizman (1 February 2008). Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment. Springer Science & Business Media. pp. 8–9. ISBN 978-1-4020-6854-6.
- ^ a b "Sepranolone - Asarina Pharma - AdisInsight".
- ^ a b Bixo M, Ekberg K, Poromaa IS, Hirschberg AL, Jonasson AF, Andréen L, Timby E, Wulff M, Ehrenborg A, Bäckström T (2017). "Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist Sepranolone (UC1010)-A randomized controlled trial". Psychoneuroendocrinology. 80: 46–55. doi:10.1016/j.psyneuen.2017.02.031. PMID 28319848.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction