
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydro-1H-inden-1-one | |
| Other names
α-Hydroindone
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 507957 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.337 |
| EC Number |
|
| 142414 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H8O | |
| Molar mass | 132.162 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 38–42 °C (100–108 °F; 311–315 K) |
| Boiling point | 243–245 °C (469–473 °F; 516–518 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
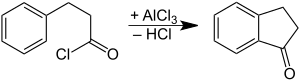
1-Indanone is the organic compound with the formula C6H4(CH2)2CO. It is one of two isomeric benzocyclopentanones, the other being 2-indanone. It is a colorless solid. 1-Indanone is a substrate for the enzyme indanol dehydrogenase.
Preparation
[edit]It is prepared by oxidation of indane or indene.[1] It can also be prepared by cyclization of phenylpropionic acid.
List of Drugs
[edit]- 2-Aminoindan synthesis (MDAI methodology using beta-keto-oxime formation with isoamylnitrite followed by reduction)
- Drinidene
- LNK-121[2]
- Pirandamine
- Pyrophendane
- SKF Derivatives[3][4]
- Indane analog of nisoxetine or Prozac.[5]
References
[edit]- ^ R. A. Pacaud, C. F. H. Allen (1938). "α-Hydroindone". Org. Synth. 18: 47. doi:10.15227/orgsyn.018.0047.
- ^ Peter T. Lansbury, Jr. Craig J. Justman, US20100292292 (2010 to Link Medicine Corp)
- ^ Ganellin, C. R.; Loynes, J. M.; Ridley, H. F.; Spickett, R. G. W. (1967). "Compounds Affecting the Central Nervous System. IV. Substituted 2-Benzyl-3-dialkylaminoalkylindenes and Related Compounds". Journal of Medicinal Chemistry. 10 (5): 826–833. doi:10.1021/jm00317a016.
- ^ Jack David, Spickett Robert Geoffr William & Ganellin Charon Robin, U.S. patent 3,159,634 (1964 to Smith Kline and French Laboratories Ltd).
- ^ Jules Freedman, U.S. patent 5,149,714 (1992 to Aventis Inc)
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction