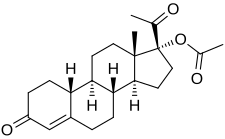
 | |
| Clinical data | |
|---|---|
| Other names | Gestronol acetate; Norhydroxyprogesterone acetate; 17α-Hydroxy-19-norprogesterone 17α-acetate; 17α-Acetoxy-19-norprogesterone; 17α-Hydroxy-19-norpregn-4-ene-3,20-dione 17α-acetate |
| Drug class | Progestin; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.242 |
| Chemical and physical data | |
| Formula | C22H30O4 |
| Molar mass | 358.478 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Gestonorone acetate, or gestronol acetate, also known as norhydroxyprogesterone acetate, is a progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups which was developed in the early 1960s but was never marketed.[1][2][3][4][5][6][7][8][9][10][11][12][13][14] It is the C17α acetate ester of gestronol (17α-hydroxy-19-norprogesterone).
Gestonorone acetate has been found to consistently inhibit ovulation at an oral dosage of 10 mg/day in combination with 50 μg/day oral ethinylestradiol.[15] Weak or no endometrial effects were observed at an oral dosage of 100 mg/day, basal vacuoles appeared at 130 to 140 mg/day, and full endometrial secretory transformation occurred at 220 mg/day.[16]
See also[edit]
References[edit]
- ^ Noguchi S (1961). "Steroids. XX. Hydrolysis of steroidal esters by malt enzyme. 1. Selective hydrolysis of steroidal acetates". Yakugaku Zasshi. 81 (3): 369–373. doi:10.1248/yakushi1947.81.3_369. ISSN 0031-6903.
- ^ Noguchi S (1961). "Steroids. XXIII Hydrolysis of steroidal esters by malt enzyme. 4. Synthesis of 17α,19-dihydroxyprogesterone and 17α-hydroxy-19-norprogesterone". Yakugaku Zasshi. 81 (3): 381–384. doi:10.1248/yakushi1947.81.3_381. ISSN 0031-6903.
- ^ Dorfman RI, Kincl FA (1963). "Steroid anti-estrogens". Steroids. 1 (2): 185–209. doi:10.1016/S0039-128X(63)80136-1. ISSN 0039-128X.
- ^ Suchowsky GK (April 1963). "Pregnancy-maintaining effect of synthetic progestogens in the rat". Acta Endocrinologica. 42 (4): 533–536. doi:10.1530/acta.0.0420533. PMID 13979052.
- ^ Kalvoda J, Heusler K, Anner G, Wettstein A (1963). "Steroids. CXCVI. 19-Norsteroids. III. Synthesis of 19-norprogesterones". Helvetica Chimica Acta. 46: 1017–1029. doi:10.1002/hlca.19630460332. ISSN 0018-019X.
- ^ Junkmann K (March 1963). "[Experimental viewpoints in the testing of synthetic gestagens]". Deutsche Medizinische Wochenschrift. 88 (13): 629–638. doi:10.1055/s-0028-1111990. PMID 13958089. S2CID 260098023.
- ^ Kincl FA, Dorfman RI (1963). "Orally active steroidal ovulation inhibitors in the adult estrus rabbit". Steroids. 2 (5): 521–525. doi:10.1016/0039-128X(63)90029-1. ISSN 0039-128X.
- ^ Suchowsky GK (1963). "Inhibition of ovulation by steroids". Journal of the Egyptian Medical Association. 1962–1963: 67–73. ISSN 0013-2411.
- ^ Junkmann (1962). "The pharmacology of new gestational and anabolic steroids". Deutsch-Englische Medizinische Rundschau. 1 (4): 385–399. ISSN 0003-3332.
- ^ Suchowsky GK, Baldratti G (September 1964). "Relationship Between Progestational Activity and Chemical Structure of Synthetic Steroids". The Journal of Endocrinology. 30 (2): 159–170. doi:10.1677/joe.0.0300159. PMID 14207040.
- ^ Nevinny-Stickel J (1964). "Inhibition of ovulation determined by estimation of pregnanediol excretion". International Journal of Fertility. 9: 57–67. PMID 14106269.
- ^ Jung H, Peters A (1967). "[The effect of various gestagens on the development of the fetus and rate of mortality in animal experiments]". Archiv Fur Gynakologie. 204 (1): 68–77. doi:10.1007/BF00668265. PMID 5630697. S2CID 22416963.
- ^ Gilbert HG, Phillipps GH, English AF, Stephenson L, Woollett EA, Newall CE, Child KJ (April 1974). "The progestational and anti-estrogenic activities of some novel 11beta-substituted steroids". Steroids. 23 (4): 585–602. doi:10.1016/0039-128X(74)90010-5. PMID 4829347.
- ^ Tang RR, Guo CC, Fan BL (2004). "Stereoselective asymmetric synthesis and characterization of 17α-acetyoxy-19-nor-progesterone". Journal of Central South University of Technology. 11 (3): 300–303. doi:10.1007/s11771-004-0061-y. ISSN 1005-9784. S2CID 195244927.
- ^ Pincus G (3 September 2013). The Control of Fertility. Elsevier. pp. 222–. ISBN 978-1-4832-7088-3.
- ^ Nevinny-Stickel J (1962). "Die gestagene Wirkung von Hydroxy-nor-Progesteronestern bei der Frau". Gewebs- und Neurohormone [The progestational effects of hydroxy-nor-progesterone esters in women]. Symposion der Deutschen Gesellschaft für Endokrinologie. Springer. pp. 248–255. doi:10.1007/978-3-642-86860-3_27. ISBN 978-3-540-02909-0.
Nach oraler Verabreichung von 100 mg des Hydroxy-nor-ProgesteronAcetats sah man nur schwache oder noch keine gestagene Wirkung am Endometrium (Abb. 3). Nach der oralen Dosis von 130- 140 mg traten basale Vacuolen auf, nach 220 mg war - außer bei einer Patientin mit individuell geringerer Ansprechbarkeit des Endometriums (2) - eine volle sekretorische Umwandlung erreicht: [...]
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction