| |

| |
| Names | |
|---|---|
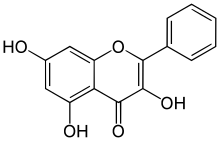
| IUPAC name
3,5,7-Trihydroxyflavone
| |
| Systematic IUPAC name
3,5,7-Trihydroxy-2-phenyl-4H-1-benzopyran-4-one | |
| Other names
Norizalpinin
3,5,7-triOH-Flavone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.147 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
| Density | 1.579 g/mL |
| Melting point | 214 to 215 °C (417 to 419 °F; 487 to 488 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Galangin is a flavonol, a type of flavonoid.
Occurrence[edit]
Galangin is found in high concentrations in plants like Alpinia officinarum (lesser galangal)[1] and Helichrysum aureonitens.[2] It is also found in the rhizome of Alpinia galanga[3] and in propolis.[4]
Biological activities[edit]
Galangin has been shown to have in vitro antibacterial[5][6] and antiviral activity.[7] It also inhibits the growth of breast tumor cells in vitro.[8][9]
References[edit]
- ^ Ciolino, H. P.; Yeh, G. C. (1999). "The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor". British Journal of Cancer. 79 (9/10): 1340–1346. doi:10.1038/sj.bjc.6690216. PMC 2362711. PMID 10188874.
- ^ Afolayan AJ, Meyer JJ (1997). "The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens". Journal of Ethnopharmacology. 57 (3): 177–181. doi:10.1016/s0378-8741(97)00065-2. PMID 9292410.
- ^ Kaur, A.; Singh, R.; Dey, C. S.; Sharma, S. S.; Bhutani, K. K.; Singh, I. P. (2010). "Antileishmanial phenylpropanoids from Alpinia galanga (Linn.) Willd" (PDF). Indian Journal of Experimental Biology. 48 (3): 314–317. PMID 21046987.
- ^ Tosi, E; Re, E; Ortega, M; Cazzoli, A (2007). "Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli". Food Chemistry. 104 (3): 1025–1029. doi:10.1016/j.foodchem.2007.01.011.
- ^ Cushnie TP, Lamb AJ (2006). "Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus". Phytomedicine. 13 (3): 187–191. doi:10.1016/j.phymed.2004.07.003. PMID 16428027.
- ^ Cushnie TP, Lamb AJ (2005). "Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss". Journal of Ethnopharmacology. 101 (1–3): 243–248. doi:10.1016/j.jep.2005.04.014. PMID 15985350.
- ^ Afolayan AJ, Meyer JJ, Taylor MB, Erasmus D (1997). "Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens". Journal of Ethnopharmacology. 56 (2): 165–169. doi:10.1016/s0378-8741(97)01514-6. PMID 917497.
- ^ So, F. V.; Guthrie, N.; Chambers, A. F.; Moussa, M.; Carroll, K. K. (1996). "Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices". Nutrition and Cancer. 26 (2): 167–181. doi:10.1080/01635589609514473. PMID 8875554.
- ^ So, F.; Guthrie, N.; Chambers, A. F.; Carroll, K. K. (1997). "Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen". Cancer Letters. 112 (2): 127–133. doi:10.1016/S0304-3835(96)04557-0. PMID 9066718.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction