| |
| Names | |
|---|---|
| Preferred IUPAC name
Iron(III) stearate | |
| Systematic IUPAC name
Iron(III) octadecanoate | |
| Other names
Iron(III) stearate, iron tristearate, ferric stearate, iron(3+) octadecanoate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.269 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C 54H 105FeO 6 | |
| Molar mass | 906.3 |
| Appearance | orange-red powder |
| Density | g/cm3 |
| Melting point | 84 °C (183 °F; 357 K) |
| Boiling point | 359.4 °C (678.9 °F; 632.5 K) |
| Hazards | |
| GHS labelling: | |
| Warning | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
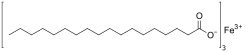
Iron(III) stearate (ferric stearate) is a metal-organic compound, a salt of iron and stearic acid with the chemical formula C
54H
105FeO
6.[2][3]
The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.[4]
Synthesis
[edit]- Reacting stearic acid with iron oxide.
- Treating stearic acid with iron chloride in presence of DABCO.[5]
Physical properties
[edit]The compound forms orange-red powder. Hygroscopic.
Insoluble in water. Soluble in hot ethanol, toluene, chloroform, acetone, benzene, turpentine.[6]
Uses
[edit]The compound is used as a catalyst in organic synthesis. Also, as a reagent in analytical chemistry, and as a stabilizer in biochemistry.[7]
References
[edit]- ^ Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 2649. ISBN 978-0-412-30120-9. Retrieved 10 March 2023.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ "Iron(III) Stearate". American Elements. Retrieved 10 March 2023.
- ^ "IRON STEARATE CAS No.555-36-2 - GO YEN CHEMICAL INDUSTRIAL CO LTD". goyenchemical.com. Retrieved 10 March 2023.
- ^ "Iron (III) Stearate | CAS 555-36-2". Santa Cruz Biotechnology. Retrieved 10 March 2023.
- ^ Basel, S., Bhardwaj, K., Pradhan, S., Pariyar, A., & Tamang, S. (2020). DBU-Catalyzed One-Pot Synthesis of Nearly Any Metal Salt of Fatty Acid (M-FA): A Library of Metal Precursors to Semiconductor Nanocrystal Synthesis. ACS Omega. doi:10.1021/acsomega.9b04448
- ^ "Iron(III) Stearate - Surfactant - SAAPedia - Surfactant Technology Platform". surfactant.top. Retrieved 10 March 2023.
- ^ "Buy Ferric stearate - 555-36-2 | BenchChem". benchchem.com. Retrieved 10 March 2023.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction