| |
| Names | |
|---|---|
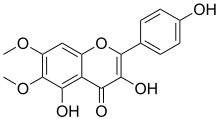
| IUPAC name
3,4′,5-Trihydroxy-6,7-dimethoxyflavone
| |
| Systematic IUPAC name
3,5-Dihydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxy-4H-1-benzopyran-4-one | |
| Other names
Betuletol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H14O7 | |
| Molar mass | 330.292 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eupalitin is an O-methylated flavonol. It can be found in Ipomopsis aggregata.[1]
Glycosides[edit]
Eupalitin 3-O-β-D-galactopyranoside can be isolated from Tephrosia spinosa.[2]
Eupalin is the eupalitin 30-rhamnoside.
References[edit]
- ^ Identification of eupalitin, eupatolitin and patuletin glycosides in Ipomopsis aggregata. D.M. Smith, C.W. Glennie and J.B. Harborne, Phytochemistry, Volume 10, Issue 12, December 1971, pp. 3115-3120, doi:10.1016/S0031-9422(00)97361-8
- ^ Eupalitin 3-O-β-D-galactopyranoside from Tephrosia spinosa. Vanangamudi A., Gandhidasan R. and Raman P. V., Fitoterapia, 1997, vol. 68, no6, p. 560, INIST 2113413
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction