| |
| Names | |
|---|---|
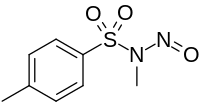
| Preferred IUPAC name
N,4-Dimethyl-N-nitrosobenzene-1-sulfonamide | |
| Other names
N-Methyl-N-nitroso-4-methylbenzenesulfonamide; N-Methyl-N-nitroso-p-toluenesulphonamide; N-Methyl-N-nitroso-4-methylbenzenesulphonamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.139 |
| EC Number |
|
| MeSH | C418734 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H10N2O3S | |
| Molar mass | 214.24 g·mol−1 |
| Appearance | Light yellow solid |
| Melting point | 61–62 °C (142–144 °F; 334–335 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Skin sensitiser, irritant, explosive[1] |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diazald (N-methyl-N-nitroso-p-toluenesulfonamide) is used as a relatively safe and easily handled precursor to diazomethane, which is toxic and unstable.[2] Diazald has become the favored commercially available precursor for the synthesis of diazomethane, compared to reagents like N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine, which are less thermally stable and more toxic and mutagenic, respectively.
Upon the addition of a base such as sodium hydroxide or potassium hydroxide and mild heating (65–70 °C) in a mixture of water, diethyl ether, and a high boiling polar cosolvent (e.g., diethylene glycol monomethyl ether[3]), the N-nitrososulfonamide undergoes successive elimination reactions to produce diazomethane (which is codistilled as an ethereal solution) as well as a p-toluenesulfonate salt as a byproduct, according to the following mechanism:

Like other nitroso compounds, it is thermally sensitive, as a result of its weak N–NO bond whose bond dissociation energy was measured to be 33.4 kcal/mol.[4]
References
[edit]- ^ External MSDS, Sigma Aldrich
- ^ Diazald in Chemical Synthesis, Sigma Aldrich
- ^ "Diazomethane". www.orgsyn.org. Retrieved 2018-07-27.
- ^ Zhu, Xiao-Qing; Hao, Wei-Fang; Tang, Hui; Wang, Chun-Hua; Cheng, Jin-Pei (March 2005). "Determination of N−NO Bond Dissociation Energies ofN-Methyl-N-nitrosobenzenesulfonamides in Acetonitrile and Application in the Mechanism Analyses on NO Transfer". Journal of the American Chemical Society. 127 (8): 2696–2708. doi:10.1021/ja0443676. ISSN 0002-7863. PMID 15725027.

Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction