 | |
| Clinical data | |
|---|---|
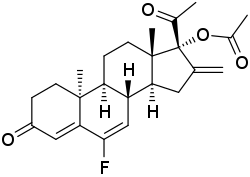
| Other names | 6-Fluoro-16-methylene-17α-acetoxy-δ6-retroprogesterone; 6-Fluoro-16-methylene-17α-hydroxy-9β,10α-pregna-4,6-diene-3,20-dione 17α-acetate; 6-Fluoro-16-methylene-3,20-dioxo-9β,10α-pregna-4,6-dien-17α-yl acetate |
| Routes of administration | By mouth |
| Drug class | Progestin; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H29FO4 |
| Molar mass | 400.490 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
DU-41165, also known as 6-fluoro-16-methylene-17α-acetoxy-δ6-retroprogesterone, is a progestin which was developed by Philips-Duphar in the 1970s and was never marketed.[1][2] It is a combined derivative of 17α-hydroxyprogesterone and retroprogesterone.[1][2] The drug shows extremely high potency as a progestogen in animals.[1] It has been found to possess 158% of the relative binding affinity of promegestone for the progesterone receptor expressed in rat uterus (relative to 74% for the closely related progestin DU-41164).[1] DU-41165 also showed 28% of the affinity of RU-28362 for the glucocorticoid receptor expressed in rat liver, but no affinity for the mineralocorticoid receptor expressed in rat kidney (<0.003% of that of RU-26752).[1] The drug showed no androgenic, anabolic, or estrogenic activity in animals, but did show some antiandrogenic and glucocorticoid activity at high doses.[1] Although highly potent in animals, DU-41165 produced little or no progestogenic effect at dosages of 50 and 200 μg/day in women, suggesting major species differences.[1] DU-41165 has been studied as a potential photoaffinity label for the progesterone receptor.[1]
References[edit]
- ^ a b c d e f g h Morsink L, de Wachter AM, Brenner P, Cekan SZ, Guerrero R, Hagenfeldt K, Diczfalusy E (May 1976). "Endocrine effects of two new retro-steroids in animal models and in women". Acta Endocrinol. 82 (1): 193–212. doi:10.1530/acta.0.0820193. PMID 57688.
- ^ a b Pinney KG, Carlson KE, Katzenellenbogen JA (February 1990). "[3H]DU41165: a high affinity ligand and novel photoaffinity labeling reagent for the progesterone receptor". J. Steroid Biochem. 35 (2): 179–89. doi:10.1016/0022-4731(90)90272-T. PMID 2308335.
| GRTooltip Glucocorticoid receptor |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PRTooltip Progesterone receptor |
| ||||||
|---|---|---|---|---|---|---|---|
| mPRTooltip Membrane progesterone receptor (PAQRTooltip Progestin and adipoQ receptor) |
| ||||||
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction