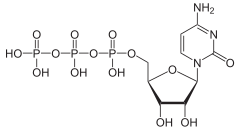
| |

| |
| Names | |
|---|---|
| IUPAC name
Cytidine 5′-(tetrahydrogen triphosphate)
| |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxyoxolan-2-yl]methyl} tetrahydrogen triphosphate | |
| Other names
CTP; Cytidine-5'-triphosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.556 |
| MeSH | Cytidine+triphosphate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H16N3O14P3 | |
| Molar mass | 483.156 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cytidine triphosphate (CTP) is a pyrimidine nucleoside triphosphate. CTP, much like ATP, consists of a ribose sugar, and three phosphate groups. The major difference between the two molecules is the base used, which in CTP is cytosine.
CTP is a substrate in the synthesis of RNA.
CTP is a high-energy molecule similar to ATP, but its role as an energy coupler is limited to a much smaller subset of metabolic reactions. CTP is a coenzyme in metabolic reactions like the synthesis of glycerophospholipids, where it is used for activation and transfer of diacylglycerol and lipid head groups,[1] and glycosylation of proteins.
CTP acts as an inhibitor of the enzyme aspartate carbamoyltransferase, which is used in pyrimidine biosynthesis.[2]
See also[edit]
References[edit]
- ^ Buchanan BB, Gruissem W, Jones RL (2000). Biochemistry & molecular biology of plants (1st ed.). American society of plant physiology. ISBN 978-0-943088-39-6.
- ^ Blackburn, G. Michael. Nucleic Acids in Chemistry and Biology. The Royal Society of Chemistry, 2006, p. 119-120.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction