| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.039.616 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| BP | |
| Molar mass | 41.7855 g/mol |
| Appearance | maroon powder |
| Density | 2.90 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) (decomposes) |
| Band gap | 2.1 eV (indirect, 300 K)[1] |
| Thermal conductivity | 4.6 W/(cm·K) (300 K)[2] |
Refractive index (nD)
|
3.0 (0.63 μm)[1] |
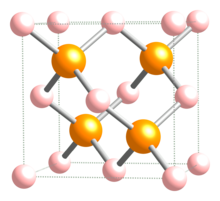
| Structure | |
| Zinc blende | |
| F43m | |
| Tetrahedral | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Boron phosphide (BP) (also referred to as boron monophosphide, to distinguish it from boron subphosphide, B12P2) is a chemical compound of boron and phosphorus. It is a semiconductor.[3]
History[edit]
Crystals of boron phosphide were synthesized by Henri Moissan as early as 1891.[4]
Appearance[edit]
Pure BP is almost transparent, n-type crystals are orange-red whereas p-type ones are dark red.[5]
Chemical properties[edit]
BP is not attacked by acids or boiling aqueous alkali water solutions. It is only attacked by molten alkalis.[5]
Physical properties[edit]
BP is known to be chemically inert and exhibit very high thermal conductivity.[2] Some properties of BP are listed below:
- lattice constant 0.45383 nm
- coefficient of thermal expansion 3.65×10−6 /°C (400 K)
- heat capacity CP ~ 0.8 J/(g·K) (300 K)
- Debye temperature = 985 K
- Bulk modulus 152 GPa
- relatively high microhardness of 32 GPa (100 g load).
- electron and hole mobilities of a few hundred cm2/(V·s) (up to 500 for holes at 300 K)
- high thermal conductivity of ~ 460 W/(m·K) at room temperature[2]
See also[edit]
References[edit]
- ^ a b Madelung, O. (2004). Semiconductors: Data Handbook. Birkhäuser. pp. 84–86. ISBN 978-3-540-40488-0.
- ^ a b c Kang, J.; Wu, H.; Hu, Y. (2017). "Thermal Properties and Phonon Spectral Characterization of Synthetic Boron Phosphide for High Thermal Conductivity Applications". Nano Letters. 17 (12): 7507–7514. Bibcode:2017NanoL..17.7507K. doi:10.1021/acs.nanolett.7b03437. PMID 29115845.
- ^ Popper, P.; Ingles, T. A. (1957). "Boron Phosphide, a III–V Compound of Zinc-Blende Structure". Nature. 179 (4569): 1075. Bibcode:1957Natur.179.1075P. doi:10.1038/1791075a0.
- ^ Moissan, H. (1891). "Préparation et Propriétés des Phosphures de Bore". Comptes Rendus. 113: 726–729.
- ^ a b Berger, L. I. (1996). Semiconductor Materials. CRC Press. p. 116. ISBN 978-0-8493-8912-2. .
Further reading[edit]
- King, R. B., ed. (1999). Boron Chemistry at the Millennium. Elsevier Science & Technology. ISBN 0-444-72006-5.
- US patent 6831304, Takashi, U., "P-N Junction Type Boron Phosphide-Based Semiconductor Light-Emitting Device and Production Method thereof", issued 2004-12-14, assigned to Showa Denko
- Stone, B.; Hill, D. (1960). "Semiconducting Properties of Cubic Boron Phosphide". Physical Review Letters. 4 (6): 282–284. Bibcode:1960PhRvL...4..282S. doi:10.1103/PhysRevLett.4.282.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction